Wound healing potential of pterospermum acerifolium wild. With induction of tumor necrosis factor - α
- *Corresponding Author:
- Aswini Kumar Senapati
Institute of Pharmacy and Technology Salipur, Cuttack, Orissa, 754202 India.
E-mail: aswinisenapati75@gmail.com
Date of Received : 11-05-2011
Date of Accepted : 01-09-2011
Available Online : 15-11-2011
Abstract
Pterospermum acerifolium, a well-known plant in Indian medicine possesses various therapeutic properties including healing properties and cytokine induction. Wound healing activity of ethanolic extract of P. acerifolium flower along with its effect on tumor necrosis factor-α (TNF-α) was assessed using excision model of wound repair in Wistar albino rats. After application of the P. acerifolium extract, rate of epithelization with an increase in wound contraction was observed. Animals tropically treated with 10% P. acerifolium extract in petroleum jelly, the wound healing process was observed faster as compared to control group which were treated with petroleum jelly alone. A significant accelerated healing was noticed in animals which were additionally prefed with 250mg/kg body weight of ethanolic P. acerifolium extract daily for 20 consecutive days along with the topical application 10% P. acerifolium extract. During wound healing phase TNF-α level was found to be up regulated by P. acerifolium treatment. Early wound healing may be pronounced due to P. acerifolium extract elevating TNF−α production.
Keywords
Cytokine induction, Pterospermum acerifolium, TNF-α, wound healing.
Introduction
Wound healing is a dynamic process of tissue restoration and re-es- tablishing the integrity of the injured skin and underlying tissues. It involves a systemic progression of events i.e. inflammation, angio- genesis, proliferation and collagen synthesis for final healing.
To restore the integrity and to avoid severe damage to the body, rapid wound healing is required. The present system of treatment by use of cortisone and other anti-inflammatory drugs may impair the healing process. Alternate method of treatment by using medicinal plants has been focused by many workers who have found the therapeutic benefits of traditional system of medicine in wound repair [1-3].
Pterospermum acerifolium Linn. has a wide application in traditional system of Indian medicine for example, in ayurvedic anticancer treatment flowers are mixed with sugars and applied locally [4]. Flowers and bark, charred and mixed with kamala applied for the treatment of small pox. Flowers made into paste with rice water used as application for hemicranias [5]. Stem bark of the plant was found to have antimicrobial activity [6]. Isolation of boscialin glucosides from leaves of P. acerifolium have been reported [7]. Hepatoprotective effect of ethanolic extract of leaves of P. acerifolium was also reported [8]. Chronic effects of P. acerifolium on glycemic and lipidemic status of type 2 model diabetic rats was found beneficial [9]. The barks are reported to be used as anti-ulcer [10] anti-inflammatory anal- gesic [11] and anti-oxidant activity [12]. Flavonoids like keampferol, keampferide, luteolin, steroids and triterpenoids like sitosterol, taraxerol, friedelin, sugars, and fatty acids are present in the plant [13,14].
In wound healing mechanism, the migration of platelets is the first response cells followed by the migration neutrophils and macrophages to the wound. Nu- merous enzymes and cytokines are secreted by macrophages and neutrophils. Among these TNF-α is the one which stimulates the angiogenesis, helps to build up the tissue granulation bed and thus has significant potential to improve the healing process [15]. In the present study, the effect of P. acerifolium flowers has been evaluated on wound healing and on TNF-α production during wound healing at different time intervals. In addition, the lipopolysaccharide (LPS) induced TNF-α production in blood of P. acerifolium treated animals has also been estimated.
Materials and Methods
Chemicals and reagents
ELISA kits for TNF-α (BD. Biosciences, USA), LPS (SIGMA chemicals, USA) and white petroleum jelly (BDH) were used.
Extract preparation
The flowers of P. acerifolium were collected from Cuttack district of Orissa, India in March, 2002. The plant was identified by the Botanical Survey of India, Howrah and a voucher specimen retained in our laboratory for future reference. The collected flowers were air-dried and pulverised using mechanical grinder.
Preparation and phytochemical study of extracts
The flowers (500 g) subjected to successive solvent extraction with 95% ethanol and water. A semi-solid extract was obtained after complete elimination of sol- vent under reduced pressure. The yield of both the extracts was 12.6±.45% and 16.2±0.32% respectively. The extracts were stored in desiccators and used for further experiment after suspending in aqueous Tween 80 solution (0.5%). The chemical constituents of the extracts were identified by qualitative chemical tests and further confirmed by thin layer chromatography study for the presence of alkaloids, sterols and flavonoids.
Animals
Inbreed Albino Wistar rats (12weeks old) of either sex weighing between 150- 180 g were obtained from the animal house Institute of Pharmacy and Technology Salipur, Cuttack, Orissa. They were housed in polyacrylic cages and fed with standard rodent pellet diet and given water ad libitum. Rats were maintained under controlled condition of light and dark cycle and temperature (24°±2°C) and tem- perature (24°±2°C). The rats were divided into 3 groups of 6 rats in each for the wounding experiment and into 2 groups of 6 rats in each for LPS experiment. All experimental protocols were approved by institutional animal ethics committee prior to the conduct of experiments.
Experimentally induced wounds
The rats were divided into three groups of 6 each. The rats were inflicted with excision wounds as described by Morton and Malone [16]. An area of 2×2 cm2 on the lateral side of thigh region, previously cleaned with soapy water was shaved and disinfected with 70% alcohol. Under local anesthesia, using 1 ml of lignocaine HCl (2%, 100mg/5ml) in depth of muscle, excision wound was created using sterile surgical blade. The entire wound was left open.
Topical application of P. acerifolium extract on wounds- Group 1 animals , served as control, were treated with petroleum jelly (vehicle) only. Animals of group 2 and 3 were topically treated with 10% P. acerifolium extract in petroleum jelly vehicle. The animals used in group 3 were pre fed daily with of P. acerifolium extract 200 mg /kg body weight orally for 20 days. The administration of the extract was continued throughout the experimental period. Healing of the wound was monitored visually up to 20-25 days (after wound formation), till the wounds completely healed in all the three groups.
Estimation of TNF-α induction in blood
Blood samples were collected by retro orbital route from the animals of all three groups at different time intervals i.e. 2, 12, 24 and 48 h after wound formation. TNF-α was determined by sandwich ELISA according to the protocol of manufactures. In brief, the ELISA plate was pre coated with anti rat TNF-α monoclonal antibodies overnight at 4°C and blocked with 3% bovine serum albumin (BSA). Plate was incubated for 2 h with different dilutions of standard TNF-α and serum samples. Anti rat TNF-α biotin conjugated monoclonal detection antibodies were added in each well and incubated for 2 h. Plate was again incubated after adding avidin –HRP conjugate for 90 min. The colour was developed by TMB and the reaction was stopped with 2N H2SO4. The reading was taken at 450-560 nm dual wavelengths. The concentrations in control and P. acerifolium treated animals were estimated by standard curve of TNF-α.
Effect of P. acerifolium extract on lipopolysaccharide (LPS) induced TNF-α production
Rats were divided into two groups of six animals each. Group A included P. acerifolium extract fed rats as described above. Group-B animals served as control. Lipopolysaccharide (LPS) 1 mg/kg body weight in 100 μl normal saline (vehicle) was injected ip. After 48h, blood was collected by retro orbital route. Collected blood was centrifuged and the serum was used for the determination of TNF-α induction by sandwich ELISA.
Statistical analysis
Results were expressed as mean ± SEM and analysed using sigmastat trial version. The statistical differences, between groups in terms of the mean of wound healing, were calculated using student’s t- test. P values < 0.05 were considered statistically significant.
Results
Effect of P. acerifolium extract on the rate of healing
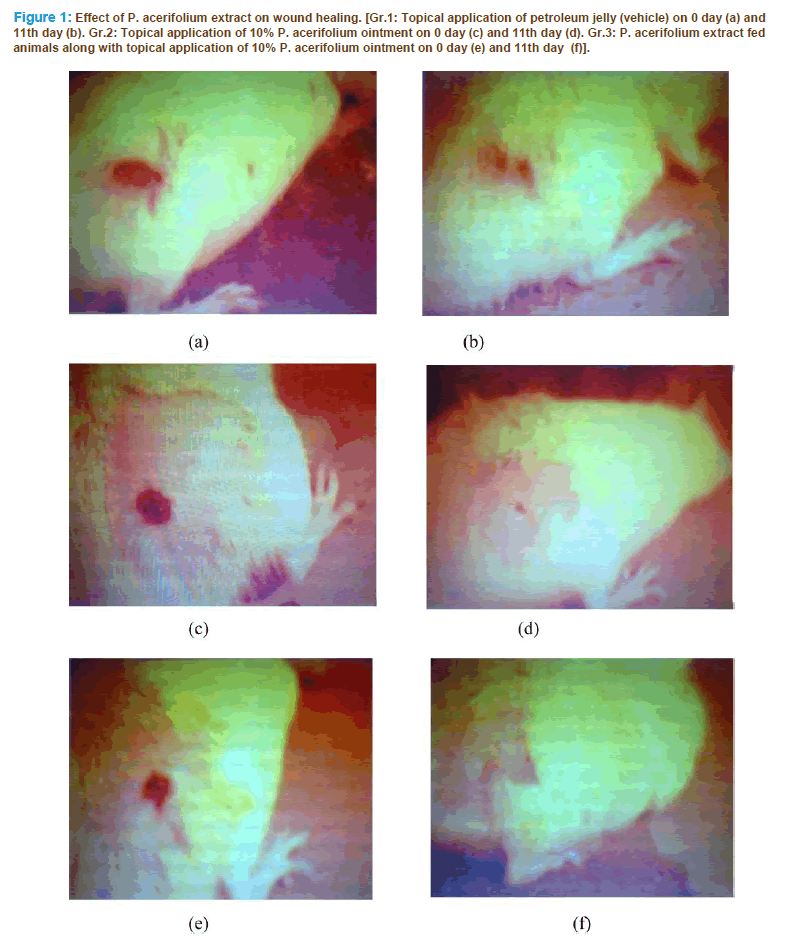
Wound inflicted on group-2 and group-3 was found to be epithelized faster in 14 and 11 days respectively as compared to control animals where the wound was healed in 20 days. Days required for healing in group-2 and group-3 were sig- nificant (P<0.001) in respect to group-1. The results indicated that P. acerifolium flower extract promoted wound repair (Figure. 1). In group-3 animals the healing was significant in respect to group-2 animals indicating the in vivo potential of P. acerifolium flower extract.
Figure 1: Effect of P. acerifolium extract on wound healing. [Gr.1: Topical application of petroleum jelly (vehicle) on 0 day (a) and 11th day (b). Gr.2: Topical application of 10% P. acerifolium ointment on 0 day (c) and 11th day (d). Gr.3: P. acerifolium extract fed animals along with topical application of 10% P. acerifolium ointment on 0 day (e) and 11th day (f)].
Effect of P. acerifolium on TNF-α production during healing
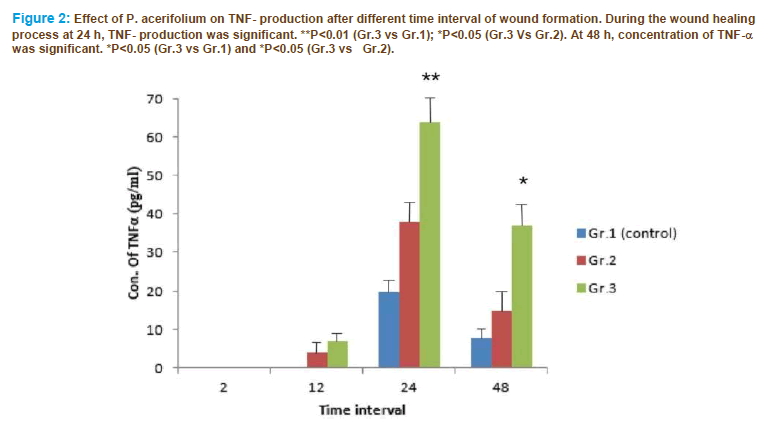
The results indicated that TNF-α level increased during 12 to 48 h. TNF-α was augmented in P. acerifolium fed rats as compared to control rats. The concentration of TNF-α induction was maximum after 24 h of wound formation and reduced after 48 h in control and topically applied, unfed animals while it sustained in P. acerifolium fed animals (Figure. 2)
Figure 2: Effect of P. acerifolium on TNF- production after different time interval of wound formation. During the wound healing process at 24 h, TNF- production was significant. **P<0.01 (Gr.3 vs Gr.1); *P<0.05 (Gr.3 Vs Gr.2). At 48 h, concentration of TNF-α was significant. *P<0.05 (Gr.3 vs Gr.1) and *P<0.05 (Gr.3 vs Gr.2).
Effect of P. acerifolium on LPS Induced TNF-α production
TNF-α production in serum was found to be significantly (P<0.001) enhanced in the P. acerifolium fed animals after 2h of LPS stimulation (from 600 pg/ml in P. acerifolium fed animals).
Discussion
The results showed that topical application of P. acerifolium in petroleum jelly, with or without oral feed of P. acerifolium, accelerated wound healing and TNF-α production. However the rats fed with P. acerifolium extract along with topical application of the extract (group-3 animals) exhibited more rapid wound heal- ing process as compared to group-2, indicating that P. acerifolium may have some ingredients for regulatory mechanism due to which there was an acceleration in wound repair. All stages of wound repair are controlled by a wide variety of different growth factors and cytokines [17,18]. Beneficial effect of many of these growth factors e.g. platelet derived growth factors (PDGFs), fibroblasts growth factors (FGFs) and granulocytes macrophages colony stimulating factors (GM-CSF) on the healing process have been demonstrated [19-22].TNF-α, a macrophage derived cytokine, is also known to play a major role in the inflammatory phase of wound healing by enhancing angiogenesis [15]. Hubner et al. [23] also revealed that during the early phase of wound repair, TNF-α was predominantly expressed in polymorphonuclear leukocytes suggesting a normal function of these cells in the initiation of wound healing.
In the present study during wound healing TNF-α was detected after 12 h of wound infliction in P. acerifolium fed animals but remained undetectable in unfed animals. After 24 h, TNF-α level increased and reached maximum in P. acerifolium fed animals. The level of TNF-α declined after 24 h of wounding in unfed animals while remained static up to 48 h in P. acerifolium extract fed animals. It is thus apparent that P. acerifolium extract, during the first stage of healing, increased the TNF-α production from polymorphonuclear leucocytes and macrophages. Hubner et al. [23] also observed a strong and early induction of TNF-α and IL- 1α and β after cutaneous injury and highest level of these cytokines were seen as early as 12-24 h after wounding. This supports the present finding that TNF-α increased up to 24 h of wounding and later down regulated after 48 h. In wound repair, expression of proinflammatory cytokines; IL-1α, IL-1β, IL-6 and TNF-α was shown to be strongly up regulated during the inflammatory phase of healing [24,25].
To support the view that up regulation of TNF-α was due to the P. acerifolium extract feeding, induction of TNF-α by LPS mitogen was also studied and it was found that after 2 h of post LPS administration there was a significant rise in TNF-α production in P. acerifolium fed rats as compared to control rats and the TNF-α level returned to its basal level with in 24 h.
However, there are reports [26,27] that TNF-α inhibits collagen formation and hydroxyproline production which are essential for the final part of prolifera- tive phase in wound healing, but the low TNF-α level after 48 h did not interfere with the collagen formation and hydroxyproline production.Antioxidant property of P. acerifolium and presence of flavonoids which scavenges the free radicals helps in the healing of wounds [12-14].
Conclusion
From the above study it was concluded that the P. acerifolium has a good wound healing potential. The accelerated healing process and induction of TNF-α by P. acerifolium extract may be the mechanisms involved in wound healing processes.
References
- Mahmood AA, Sidik K, Salmah I. Wound healing activity of Carica papaya L. aqueous leaf extracts in rats. Int J Mol Med Ad Sci. 2005; 1: 398.
- Nayak S, Nalabothu P, Sandiford S, Bhogadi V, Adogw A. Evaluation of wound healing activity of Allamanda cathartic L. and Laurusnobilis L. extracts on rats. BMC Complementary Alternative Med. 2006; 6: 12.
- Rathi BS, Bodhankar SL, Baheti AM. Evaluation of aqueous leaves extract of Maringa oleifera Linn for wound healing in albino rats. Indian J Exp Biol. 2006; 44: 898.
- Balachandran P, Govindrajan R. Cancer – An ayurvedic perspective. Pharmacol Res 2005; 51:19-30.
- Caius JF. The Medicinal and Poisonous Plants of India. Indian Medicinal Plants. Scientific publisher-Jodhpur 1990; 2: 489.
- Khond M, Bhosale JD, Arif T, et al. Screening of some selected medicinal plants extracts for in vitro anti-microbial activity. Middle-East J Sci Res 2009; 4: 271-278.
- Mamun MIR, Nahar N, Azad Khan Ali L, et al. ASOMPS X 2000, Book of Abstracts; 2000:73.
- Kharpate S, Vadnerkar G, Jain D, et al. Evaluation of hepatoprotective activity of ethanol extracts of Pterospermum acerifloium Ster leaves. Indian J Pharm Sci 2007; 69: 850-2.
- Murshed S, Rokeya B, Ali L, et al. Chronic effects of Pterospermum acerifolium bark on glycemic and lipedemic status of type 2 diabetic model rats. Diabet Res Clinical Prac. 2000; 50: 224-30.
- Manna A.K.; Behera A.K., Jena J, et al. The antiulcer activity of Pterosper- mum acerifolium barks extract in experimental animal. Journal Pharmacy Research. 2009; 2(5): 785-8.
- Manna A.K., Jena J. Anti inflammatory and Analgesic activity of bark extract of Pterospermum acerifolium. International Journal of Current Pharmaceuti- cal Research.2009; 1(1):32-7.
- Manna AK, Manna S, Behera AK, Kar S. In vitro antioxidant activity of P. acerifolium barks. Journal Pharmacy Research. 2009; 2(6): 1042-4.
- Harborne JB. Phytochemical Methods- A guide to modern techniques of plant analysis. 3rd ed. Chapman and Hall, 1998:56, 81-3, 92-6,115-20.
- The Wealth of India. J-Q, CSIR, N. Delhi, 2003; 4: 423-24.
- Rosenberg LZ. Wound healing, growth factors. Emedicine, 2009. available at: http://emedicine.medscape.com/article/1298196-overview. Accessed May 27, 2009
- Morton JJ, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Inter Pharmacodyn. 1972; 176: 117.
- Martin P. Wound healing aiming for perfect skin regeneration, Science, 1997; 276: 75.
- Werner S, Grose R. Regulation of wound healing by growth factors and cy- tokines. Physiol Rev. 2002; 83: 835.
- Abraham JA, Klagsbrun M. Modulation of wound repair by members of the fibroblast growth factor family In: The molecular and cellular biology of wound repair. Clark RAF, ed. 2nd ed. Plenum, New York. 1996: 195.
- Greenhalgh DG. The role of growth factors in wound healing. J Trauma. 1996; 41: 159.
- Harding KG, Morris HL, Patel GK. Science, medicine and the future: Healing chronic wounds. Br Med J. 2002; 324: 160.
- Nath C, Gulati SC. Role of cytokines in healing chronic skin wounds. Acta Haematol, 1998; 99: 175.
- Hubner G, Brauchle M, Smola H, et al. Differential regulation of pro-inflam- matory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996; 8: 548.
- Grellner W, Georg T, Wilske J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-α) in human skin wounds. Forensic Sci Int, 2000; 113: 251.
- Grose R, Werner S, Kessler D, et al. A Role for endogenous glucocorticoids in wound repair, EMBO Reports, 2002; 3: 575.
- Rapala K. The effect of tumor necrosis-alpha on wound healing, an experi- mental study. Ann Chir Gynacol Suppl. 1996; 211: 1.
- Buck M, Houglum K, Chojkier M. Tumor necrosis factor-alpha inhibits col- lagen alpha 1(I) gene expression and wound healing in a murine model of cachexia. American J Pathology. 1996; 149: 195.