Role of liquid membrane phenomenon in the anti-bacterial activity of Cefuroxime Sodium
- *Corresponding Author:
Date of Received: 16-02-2010
Date of Modified: 10-03-2010
Date of Accepted: 23-04-2010
Available Online: 15-05-2010
Abstract
The role of liquid membrane phenomenon has been studied in the anti bacterial activity of cephalosporins i.e. Cefuroxime sodium. In our earlier publica-tion [1] it was reported that hydraulic permeability data obtained to demonstrate the existence of liquid membrane in series with supporting membrane generated by Cefuroxime sodium. Transport of selected permeants (glucose, PABA, glycine, and ions like Mg++, NH4+, PO4-, Ca++, Na+, K+ and Cl-) through liquid membrane generated by Cefuroxime sodium in series with supporting membrane has been studied. The results indicated that the liquid membrane generated by Cefuroxime sodium inhibit the transport of various essential bio-molecules and permeants in to the cell. This modification in permeability of different permeants in the presence of the liquid mem-branes is likely to play significant role in the biological actions of Cefuroxime sodium. The anti-bacterial activity of Cefuroxime sodium further confirmed that the generation of liquid membrane by Cefuroxime sodium is also contributing for the antibacterial activity of them.
Keywords
Cefuroxime sodium, liquid membrane, hydraulic permeability, solute permeability, anti-microbial activity
Introduction
Many Pharmacologically active compounds are amphiphillic in nature, which may undergo different types of association, and whose site of action is frequently, the Plasma membrane. In many cases excellent correlation between surface activity of drugs and their biological actions was demonstrated. Earlier reports shows that the wide variety of surface active drugs reveled that there might be a common mechanism due to surface activity, which governs their action [2-12]. It has been shown that the surface-active drugs at the site of their action generate liquid membranes, which acts as barrier to the transport of relevant permeants. There are reports that many antmicrobial agents like Norfloxacin, Ciprofloxacin [13], Cefuroxime sodium [1] and Tinidazole[14] are amphiphillic in nature and generate the liquid membrane at the site of their action. In addition they have reported that this phenomenon also contribute for their anti-microbial action.
In the present study Cefuroxime sodium, (Fig. 1) a second generation broad-spectrum cephalosporin was used, having both hydrophilic and hydrophobic domains in its structure. In our earlier publication it was reported that Cefuroxime sodium at critical micellar concentration (CMC) form the liquid membrane over the cytoplasmic membrane of bacteria. Because of the liquid membrane formed by Cefuroxime sodium the transport of essential ions, glucose and p-amino benzoic acid was decreased. Cellulose Acetate Microfiltration membrane has been specifi- cally chosen as the site for formation of liquid membrane. The present study is designed so as to assess this hypothesis and its role in the anti-microbial activity of the study drug.
Materials and Methods
Materials
Cefuroxime sodium (KAPL Bangalore), glucose A.R, calcium chloride AR, sodium chloride, ammonium chloride, magnesium sulphate, potassium dihydrogen phosphate(Qualigens Chemicals pvt Ltd, Mumbai), para amino benzoic acid (Sd fine Chemicals Ltd., Mumbai), lecithin (Genuine chemicals Co. Mumbai), cholesterol (Rolex laboratories, Mumbai), peptone (Sd fine Chemicals Ltd., Mumbai), beef extract (Qualigens Chemicals, Mumbai), agar (Sd fine Chemicals Ltd., Mumbai), dextrose (Sd fine Chemicals Ltd., Mumbai), casein hydroxylate of soyabean (Burgoyne Burbiges and Co., India, Mumbai), yeast extract (Loba Chemicals Pvt Ltd., Mumbai), triple Distilled Water. The chemicals used were of AR grade.
Methods
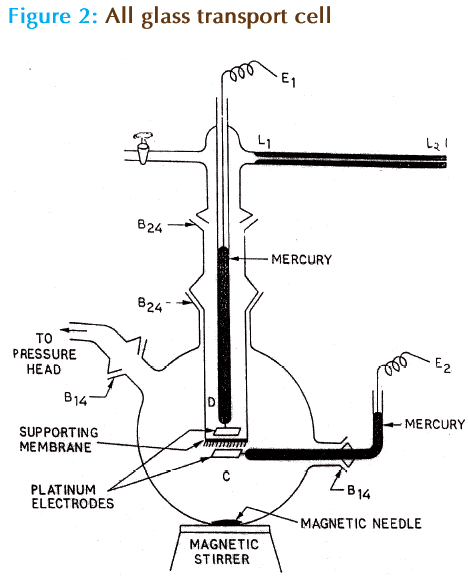
The Critical Micellar Concentration (CMC) of aqueous Cefuroxime sodium was determined from the variation of surface tension with the concentration at 37 ± 0.1 °C and was found to be 5 × 10-4 (Table-1). Surface tensions were measured using a model–144 Du Nouy surface tensiomat (Komal scientific Co., Bombay–47). The all glass transport cell as shown in fig.2 [1-13] was used to obtain hydraulic permeability and solute permeability data. It essentially consists of two components, C and D separated by Sartorius cellulose acetate micro filtration membrane (Cat. No.11107, pore size 0.2μm, thickness 1 × 10-4m, area 2.55 × 10-5m2), which acts as a supporting membrane for the liquid membrane. The cellulose acetate membrane was coated with lecithin (1.919 × 10-5M) and lecithin-cholesterol (1.919 × 10-5M lecithin and 1.175 × 10-6M cholesterol) so as to simulate the bacterial and human cell membranes respectively. For the measurement of hydraulic permeability data, aqueous solution of Cefuroxime sodium at various concentrations were placed in compartment C and compartment D was filled with de-ionized water. The concentration ranges of Cefuroxime sodium were so chosen that covers both above and below CMC. The hydraulic permeability was determined separately for Sartorius cellulose acetate micro filtration membrane coated with lecithin alone and cholesterol + lecithin. The procedure described in the earlier publication [9] was adopted for obtaining the hydraulic permeability data.
| Serial No. | Molar concentration (M) | Concentration (μg/ml) | Surface Tension (dy/cm) |
|---|---|---|---|
| 01 | 1 × 10-3 | 04.463 | 67.735 ± 0.350 |
| 02 | 2 × 10-3 | 08.926 | 69.190 ± 0.364 |
| 03 | 3 × 10-3 | 13.389 | 69.852 ± 0.360 |
| 04 | 4 × 10-3 | 17.852 | 70.507 ± 0.357 |
| 05 | 5 × 10-3 | 22.315 | 70.181 ± 0.000 |
| 06 | 6 × 10-3 | 26.778 | 69.742 ± 0.339 |
| 07 | 7 × 10-3 | 31.241 | 69.523 ± 0.000 |
| 08 | 8 × 10-3 | 35.704 | 68.522 ± 0.367 |
| 09 | 9 × 10-3 | 40.167 | 67.735 ± 0.350 |
| 10 | 1 × 10-4 | 00.446 | 68.969 ± 0.271 |
| 11 | 2 × 10-4 | 00.892 | 64.249 ± 0.369 |
| 12 | 3 × 10-4 | 01.338 | 58.232 ± 0.414 |
| 13 | 4 × 10-4 | 01.785 | 56.211 ± 0.950 |
| 14 | 5 × 10-4 | 02.231 | 54.073 ± 0.766 |
| 15 | 6 × 10-4 | 02.677 | 61.190 ± 0.305 |
| 16 | 7 × 10-4 | 03.124 | 62.922 ± 0.609 |
| 17 | 8 × 10-4 | 02.570 | 63.649 ± 0.396 |
| 18 | 9 × 10-4 | 04.016 | 66.362 ± 0.357 |
The values are presented as arithmetic mean ± standard deviation of 6 determinations
Table 1: The critical micellar concentration (CMC) of aqueous solution of Cefuroxime sodium [1]
Where Js and Jv are the solute flux and volume flux per unit area of the membrane, respectively. Δπ is the Osmotic pressure difference across the membrane. For the measurement of ω, one compartment of the transport cell (2CMC) was filled with aqueous solution of Cefuroxime sodium along with permeant. Other compartment was filled with de-ionized water. In control experiments, no drug was used; concentration of the drug in these experiments was always kept higher than its CMC to ensure complete coverage of the supporting membrane with the liquid membrane generated by Cefuroxime sodium. All measurements were made at constant temperature (37 ± 0.1 °C) using a thermostat.
Study of antimicrobial activity
Cefuroxime sodium a second generation cephalosporins was screened against bacteria at above CMC, CMC and below CMC (0.25, 0.5, 0.75, 1.00, 2.00 and 3.00 CMC) to know their antimicrobial activity in these concentrations. To screen Cefuroxime sodium for antibacterial activity, bacteria like staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative) were chosen for the present study. The antibacterial activities was performed by cup plate method (diffusion technique).
Results and discussion
In our earlier publication [1] it was reported that Critical Micellar Concentration (CMC) of aqueous Cefuroxime sodium was determined from the variation of surface tension with the concentration at 37 ± 0.1 °C and was found to be 5 × 10-4 (Table-1).
The hydraulic permeability data at various concentrations of Cefuroxime sodium were found to obey the linear relationship i.e.
Jv = Lp. Δp. ---------- (2)
Where, Jv represents the volume flux per unit area of the membrane, Δp is the applied pressure difference and Lp is the hydraulic conductivity coefficient. Values of Lp at various concentrations of Cefuroxime sodium were obtained from a plot Jv Vs Δp (slope of the plot is Lp). The value shows decreasing trend with increasing concentration of Cefuroxime sodium up to CMC. Beyond which it becomes more or less constant. This is indicative of progressive coverage of the supporting membrane with liquid membrane generated by the drug in accordance with the Kesting hypothesis [15].
Analysis of hydraulic permeability data in the light of mosaic membrane model [16-17] further supports the existence of the liquid membrane in series with supporting membrane. Following the arguments given earlier it can show that concentration of the surfactant is n’ times its CMC, n being less than or equal to 1, the value of Lp would be equal to [(1-n) Ls p + n Lc p], where Ls p and Lc p represents the value of Lp at 0 and the CMC of the surfactant respectively. The values of Lp thus computed for 0.25CMC, 0.5CMC and 0.75CMC of Cefuroxime sodium are in good agreement with the experimentally determined values.
The hydraulic permeability data using aqueous mixtures of lecithin (1.919 × 10-5M) alone, lecithincholesterol mixture and with drug was conducted. It was earlier reported that lecithin and lecithincholesterol aqueous solutions form liquid membrane, which completely covers the supporting membrane indicate the fall in Lp values when 1 CMC solution was added to the compartment C, which provides additional evidence regarding incorporation of Cefuroxime sodium in lecithin and lecithin-cholesterol membranes [9-10].
From the solute permeability (ω) data it was observed that Cefuroxime sodium reduce the permeation of D-Glucose, PABA and ions like magnesium, phosphate, ammonium, Sodium, Potassium, Calcium and Chloride [1]. It seems Cefuroxime sodium forms the liquid membrane over the cell membrane and inhibits the transport of the essential ions and bio-molecules and thereby inhibits the normal functioning of cell. This may also contribute for the bactericidal effect of the Cefuroxime sodium. In addition to this the permeability of various solutes through lecithin + cholesterol membrane is also reduced significantly. This may contribute for the side effects associated with the drug. However, further studies are needed to confirm this.
In the present investigation it was observed antimicrobial activity (Table-2) of the study drug at 0.25, 0.5, 0.75, 1.0, 2.0 and 3.0 CMC was studied against Gram-positive and Gram-negative organism. It was found that the zone of inhibition of Cefuroxime sodium against S.aureus and E.coli increased upon increase in concentration up to 1 CMC and above CMC it was almost constant. The data of antibacterial activity of Cefuroxime sodium was indicated that the CMC and MIC of Cefuroxime sodium are nearer or within the effective range. These results further confirmed that the generation of liquid membrane by these agents is also contributing for the antibacterial activity of Cefuroxime sodium. Hence, we can suggest that the liquid membrane generated by Cefuroxime sodium do contribute for antibacterial activity as a complementary to receptor specific mechanisms explained earlier.
| Sl. No. | Critical Micellar Concentration (CMC) | Concentration (μg/ml) | Zone of Inhibition (mm) | |
|---|---|---|---|---|
| S.aureus | E.coli | |||
| 01 | 0.25 | 0.56 | 7.233 ± 0.404 | 6.000 ± 0.000 |
| 02 | 0.50 | 1.12 | 8.083 ± 0.144 | 8.200 ± 0.346 |
| 03 | 0.75 | 1.78 | 10.333 ± 0.577 | 14.166 ± 0.288 |
| 04 | 1.00 | 2.23 | 16.333 ± 0.577 | 18.166 ± 0.288 |
| 05 | 2.00 | 4.46 | 17.000 ± 0.000 | 20.333 ± 0.577 |
| 06 | 3.00 | 6.69 | 17.000 ± 0.000 | 21.666 ± 0.577 |
The values are presented as arithmetic mean ±standard deviation of 6 determinations.
Table 2: Antimicrobial activity at various concentrations of Cefuroxime sodium
Conclusion
It is apparent from the results that the generation of liquid membrane over the cell membrane may also contribute for the bactericidal effect of Cefuroxime sodium. Antibacterial activity of Cefuroxime sodium at below CMC, CMC and above CMC against S.aureus and E.coli indicates that as the concentration of these drug increases the antibacterial activity also increases at above CMC the antibacterial activity is more or less constant. This confirms the liquid membrane formed by Cefuroxime sodium will act as an additional mechanism to the conventional mechanism.
Acknowledgements
The authors are highly indebted to the President and Secretary, TMAE Society, Harapanahalli for their support.
References
- Nagesh C, Venkatesh JS, Shankaraiah MM, et al. Role of liquid membrane hypothesis in the mechanism of action cefuroxime sodium. Orien. J. Chem. 2008; 24(1): 271-76.
- Bhise SB, Marwadi PR, Mathur SS, et al. Liquid membrane phenomena in haloperidol action, J. Pharm. Sci. 1982: 71: 526-29.
- Bhise SB, Marwadi PR, Mathur SS, et al. Liquid membrane phenomenon in reserpine action, J. Pharm. Sci. 1983: 72: 599-01.
- Bhise SB, Subrahmanyam CVS and Srivastava RC, Liquid membrane phenomenon in antihistamines, Int. J. Pharm. 1983; 72: 263-72.
- Bhise SB, Bhattacharajee D and Srivastava RC, Liquid membrane phenomena in diuretics, J. Pharm. Sci. 1984; 73: 1579-81.
- Bhise SB, Subrahmanyam CVS, Mathotra AK, et al. Liquid membrane phenomenon in antiepileptic drugs, Int. J. Pharm. 1985: 24: 297-05..
- Bhise SB, Subrahmanyam CVS, Sharma RK, et al. Liquid membrane in anti-arrhythmic action, Int. J. Pharm. 1986; 28: 145-49.
- Srivastava RC and Jakhar RPS, Transport through liquid membranes generated by lecithin-cholesterol mixtures, J. Phy. Chem. 1981; 85: 148.
- Srivastava RC and Jakhar RPS, Transport through liquid membranes generated by lecithin-cholesterol mixtures, J.Phy.Chem. 1982; 86:1441-45.
- Srivastava RC, Bhise SB and Mathur SS, Liquid membrane phenomena and drug action, Adv colloid Interface Sci. 1984; 20: 131-61.
- Srivastava RC, Raju DB, Singh V, et al. Transport through liquid membranes generated by lecithin, cholesterol and lecithin-cholesterol mixture in presence of prostaglandins, J. Memb. Sci. 1991; 58: 211-20.
- Srivastava RC, Sharma RK, Tandon A, etal. Transport through liquid membrane bilayers generated by lecithin, cholesterol and lecithin-cholesterol mixture studies in the presence of polyene antibiotics, J. Colloid Interface Sci. 1985; 108: 249-56.
- Nagappa AN, Kole PL, Pandi PV, et al. Transport studies through liquid membranes of ciprofloxacin and norfloxacin, Ind J. Biochem Biophys. 2004; 41: 48–52.
- Nagesh C, Venkatesh JS, Shankraiah MM, et al. Role of liquid membrane phenomenon in the biological actions of Tinidazole. Pharmacologyonline. 2009; 1: 518-32.
- Kesting RE, Subcasky WJ and Paton JD. Liquid membrane at the cellulose acetate membrane/saline solution interface in reverse osmosis, J. Colloid Interface Sci. 1968; 28: 156-60.
- Katchalsky A and Curran PF. Non-equilibrium Thermodynamics in Biophysics, Harvord University Press, Cambridge M.A,1967
- Katchalsky A and Kedem O. Thermodynamics of flow processes in biological systems, Biophys J. 1962; 2: 53-78..