Rofecoxib prevents ctdsDNA against damage induced by copper sulfate and ultraviolet B radiation in vitro study
- *Corresponding Author:
- Marwan S. M. Al-Nimer
Department of Pharmacology, College of Medicine, Al-Mustansiriya University, P.O. Box 14132, Baghdad, Iraq
E-mail: alnimermarwan@ymail.com
Date of Received :27-06-2010
Date of Modified :06-12-2010
Date of Accepted :11-12-2010
Available Online :15-02-2011
Abstract
Rofecoxib is a selective cyclooxygenase COX-2 enzyme inhibitor with chemoprotective effect against cancer in experimental models. This study aimed to investigate the effect of rofecoxib against ctds DNA damage induced by copper ions or ultraviolet (UV)B radiation. Aliquot ctdsDNA samples were incubated with copper sulfate solution (50 nmol) and rofecoxib (0.8 mol) was added either before or after the admixing the ctdsDNA with copper sulfate. In another experimental series, aliq-uot of ctdsDNA were exposed to UVB radiation for 30 min in absence or presence of rofecoxib. Rofecoxib significantly attenuated the separation of double strands of DNA (detected by increase the absorbance of DNA at 260 nm) induced by Cu ions. Rofecoxib significantly offered protection against UVB-induced DNA damage. It is concluded that rofecoxib offered protection against copper ions or UVB induced-DNA damage via different mechanisms not related to the inhibition COX-2.
Keywords
Rofecoxib, ctdsDNA, UVB
Abbreviations
COX: cyclooxygenase ctdsDNA : calf thymus double strand deoxyribonucleotide CuSO4: copper sulfate UVB: ultraviolet B UVC: ultraviolet C
Introduction
Rofecoxib, a nonsteroidal anti-inflammatory drug, has a greater selectivity for cyclo-oxygenase (COX)-2 isoenzyme with little or no effect on the COX-1 isoenzyme [1]. Previous studies reported a 50% reduction in serious gastrointestinal outcomes, but a 5-fold increase in thromboembolic cardiovascular events [2-4].
In 2004, Merck withdrew rofecoxib from the market after its Adenomatous Polyp Prevention on Vioxx (APPROVe) trial showed a 2-fold increase in cardiovascular risk with 25 mg/d of rofecoxib compared with placebo [5]. COX-2 inhibitors seem to have a chemoprotective effect on colorectal cancer in the general population, but it is still used in some countries [6,7] Wood et al [8] reported that rofecoxib inhibited endometrial cancer cell proliferation via unknown mechanism by the evidences that it did induce apoptosis, alterations of the cell cycle, or changes in mismatch repair gene expression as with aspirin. In vitro study using the single-cell gel electrophoresis (Comet) assay, COX-2 inhibitors expressed direct antimutagenic effect by reducing DNA strand-breaks in pharyngeal mucosa cells treated with hydrogen peroxide.[9] On the other hand, Kusunokiet al found that COX-2 inhibitors induced DNA fragmentation and caused a marked decrease of synovial fibroblast cell viability from patients with rheumatoid arthritis and osteoarthritis i.e. they had a proapoptotic effect [10].
In human keratinocyte cell line, ultraviolet UVB radiation induced COX-2 and prostaglandin E2 production through a nuclear factor-kappaBdependent pathway [11]. Nimesulide, a selective COX-2 inhibitor reduced the growth of UVBinduced tumors both in terms of tumor number and tumor volume as well as inhibited the malignant progression of squamous cell carcinoma of skin in mice irradiated with UVB twice weekly for 35 weeks [12].
Therefore it is interesting to explore the direct effect of rofecoxib, a selective COX-2 inhibitor against ctds DNA damage induced by copper sulfate or UVB radiation.
Materials And Methods
Th is study is conducted at department of pharmacology and department of physiology/medical physics, college of medicine, Al-Mustansiriya university in cooperation with department of physiology/ medical physics, Diala university in Iraq. All the experiments were done on the purified ctdsDNA of molecular weight calculated from SV20, w was 3.56 x103g/mol, purchased from BDH chemicals, England. Known weigh of DNA was dissolved in isotonic citric solution (0.0015M sodium chloride, 0.00015 trisodium citrate) and different concentrations were prepared for the following experiments:
1. Effect of copper sulfate (CuSO4) on aliquots ctdsDNA samples. An aliquot of CuSO4 (final concentration 50 nmol) as oxidizing agent was added to the aliquots of ctdsDNA samples (5μg/mL), incubated at room temperature for 10 minutes. The absorbance (O.D.) of five samples for each treatment as well as non treated samples served as control were recorded at λ 260 nm using UV-visible spectrophotometer.
2. Effect of rofecoxib on aliquot ctdsDNA samples in presence or absence of copper sulfate. Rofecocib, dissolved in methanol (final concentration 0.8μmol), was added to aliquot mixture of ctdsDNA (5μg/mL) and 50 nmol CuSO4 (final concentration, and then incubated at room temperature for 10 minutes (treatment 1). Further experiments were done by adding CuSO4 solution to the aliquot mixture of ctdsDNA and rofecoxib, and then incubated for 10 minutes (treatment 2). Control experiments were carried on using an equivalent volume of methanol instead of rofecoxib. The absorbance (O.D.) of five samples for each treatment as well as non treated samples served as control were recorded at λ 260 nm using UV-visible spectrophotometer.
3. Effect of ultraviolet B radiation on the aliquot ctdsDNA. ctdsDNA solutions (final concentration (10μg/mL) were exposed to UVB radiation for several interval periods (10, 20, 30, 40, 50, 60 minutes). The ultraviolet light source is provided by the EL series ultraviolet lamps. The lamp utilizes 8 watts, wavelength 302 nm dual bi-pins tube, plugged into the service outlet located on the top of the cabinet of 20cm x 8cm x 15cm. of five samples for each treatment as well as non irradiated samples served as control were recorded at λ 260 nm using UV-visible spectrophotometer.
4. Effect of rofecoxib on aliquot ctdsDNA samples exposed to UVB radiation. An aliquot samples of ctdsDNA (10μg/mL) treated with rofecoxib (0.8μmol) or methanol were exposed to UVB radiation for 30 minutes. Then the absorbance (O.D.) of five samples for each treatment as well as non irradiated samples served as control were recorded at λ 260 nm using UV-visible spectrophotometer.
All of the chemicals were of analar grades. Rofecoxib is a gift from Dofar pharmaceuticals, Baghdad, Iraq. It dissolved in methanol and prepared freshly prior to each experiment.
The results are expressed as absolute number, percentage, and mean ± SD of number of observations. The data were analyzed using student's t test taking p ≤ 0.05 as the lowest limit of significance.
Results And Discussion
Table 1 shows that CuSO4 induced significant (p < 0.001) increase in absorbance (O.D.) of ctdsDNA at λ 260 nm. Methanol treatment did not show significant effect on the CuSO4-induced ctdsDNA damage. Addition of rofecoxib either prior to or concomitantly with CuSO4 significantly attenuated the CuSO4-induced ctdsDNA damage by 11.9% and 7.6% respectively compared with 19.6% ctdsDNA damage without rofecoxib. Furthermore, rofecoxib produced significant protection against CuSO4 induced ctdsDNA damage when it incubated with ctdsDNA solution prior to the addition of CuSO4 compared with concomitant addition of rofecoxib and CuSO4 to the ctdsDNA solution. UVB-radiation significantly increased the absorbance (O.D.) of ctdsDNA solution.
| Treatment | Absorbance (O.D.) of ctdsDNA solution at λ 260 nm | |
|---|---|---|
| Without CuSO4 | With CuSO4 | |
| CtdsDNA solution | 0.0652 ± 0.00109 | 0.0780 ± 0.00209* |
| CtdsDNA solution + Methanol | 0.0670 ± 0.00141 | 0.0820 ± 0.00288* |
| CtdsDNA solution + Rofecoxib (Treatment 1) | 0.0654 ± 0.00207 | 0.0732 ± 0.00746*† |
| CtdsDNA solution + Rofecoxib (Treatment 2) | 0.0654 ± 0.00207 | 0.0704 ± 0.00907*†‡ |
The results are expressed as mean ± SD of (n=5). * p < 0.001 compared with corresponding group without CuSO4 treatment. † p < 0.001 compared with ctdsDNA solution treated CuSO4. ‡ p < 0.001 compared with rofecoxib (treatment 1).
Table 1: The effect of rofecoxib (0.8 μmol)on the ctdsDNA (5 μg/mL) solution challenged with copper sulfate (50nmol).
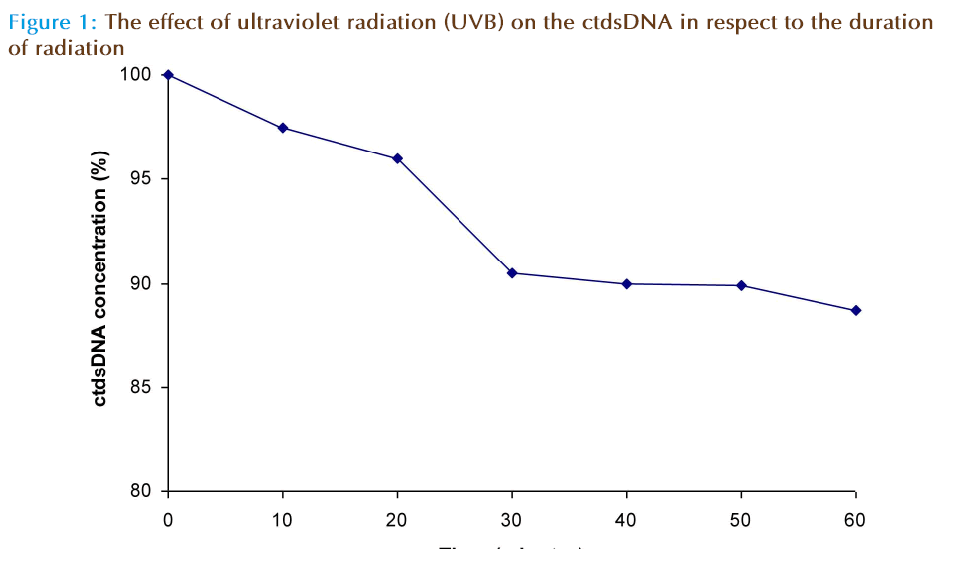
Figure 1 shows that the percent of ctdsDNA damage did not show linear correlation with the radiation period. The changes in the absorbance(O.D.) of ctdsDNA solution during the interval periods 30 to 60 minutes remained constant.
Methanol did not offer significant effect on the UVB radiation-induced ctdsDNA damage while rofecoxib offered significant protection against UVB radiation (Table 2). The absorbance (O.D) of irradiated ctdsDNA solution in presence of rofecoxib matched that of non-irradiated ctdsDNA solution in absence of rofecoxib.
| Treatment | Non irradiated | Irradiated |
|---|---|---|
| CtdsDNA solution | 0.1146 ± 0.0029 | 0.1253 ± 0.0015* |
| CtdsDNA solution + Methanol | 0.1153 ± 0.0025 | 0.1267 ± 0.0011* |
| CtdsDNA solution + Rofecoxib | 0.1150 ± 0.0022 | 0.1152 ± 0.0010† |
The results are expressed as mean ± SD of (n=5). * p < 0.001 compared with corresponding non irradiated group. † p < 0.001 compared with irradiated ctdsDNA solution in absence or presence of methanol.
Table 2: The effect of rofecoxib (0.8 μmol) on the ctdsDNA(10 μg/mL) solution irradiated by UVB light for 30 minutes.
The results of this study show the direct chemopreventative effect of rofecoxib against DNA damage induced by chemical agent and ultraviolet radiation. The effects of CuSO4 and UVB radiation against ctdsDNA solution are similar. Both of them increased the absorbance of ctdsDNA which indicates separation of the DNA strands i.e. hyperchromasia effect [13]. It is well known that Cu ions act as catalyst in the cleavage of DNA strands [14]. In this work, Cu ions act directly , neither as a catalyst nor generate free radical in aqueous solution as with UV radiation [15].
Rofecoxib attenuates the separation of the DNA strands induced by CuSO4 and prevents the separation induced by UVB. Therefore, the effect of rofecoxib against CuSO4 induced DNA damage differs from that induced by UVB radiation. The possible explanation for these findings that rofecoxib, in vitro, chelates or prevent the Cu ions to attack the DNA molecule i.e. antagonist effect. The results of Nagano and Bush are in contradictory with this explanation who found that the prostaglandin E2 production is enhanced by COX-2 after exposure to Cu [16]. The findings of Viossat et al are in agreement with the present study, which demonstrated by X-ray diffraction methods the Cu(II) chelates indomethacin, a non selective COX inhibitor [17].
In the UVB-radiation induced-DNA damage, rofecoxib acts as free radicals scavenger in protection the DNA [9]. Previous studies reported that rofecoxibs reduces the reactive oxygen and nitrogen species in different experimental models [18,19]. The limitations of this study include; determination of effective protective concentration (EC50) of rofecoxib, and to explore the effect of rofecoxib against DNA damage induced by UVA and UVC.
Conclusion
It concludes that Rofecoxib offered protection against copper ions or UVB induced-DNA damage via different mechanisms.
References
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001; 345: 433-442.
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000: 343: 1520-1528.
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase-2. JAMA 2006; 296: 1633-1644.
- van Staa TP, Smeeth L, Persson I, et al. What is the harm–benefit ratio of Cox-2 inhibitors? Int J Epidemiol 2008; 37: 405-413.
- Merck and Co Inc: Merck announces voluntary worldwide withdrawal of Vioxx [news release; September 30, 2004]. http://www. merck.com/newsroom/vioxx/pdf/vioxx_press_release_final.pdf. Accessed August 4, 2006.
- Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 2006; 131:1674-1682.
- Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009; 17: 55-67.
- Wood NJ, Quinton NA, Burdall S, et al. Exploring the potential chemopreventative effect of aspirin and rofecoxib on hereditary nonpolyposis colorectal cancer-like endometrial cancer cells in vitro through mechanisms involving apoptosis, the cell cycle, and mismatch repair gene expression. Int J Gynecol Cancer 2007; 17: 447-454.
- Matthias C, Schuster MT, Zieger S et al. COX-2 inhibitors celecoxib and rofecoxib prevent oxidative DNA fragmentation. Anticancer Res 2006; 26(3A): 2003-2007.
- Kusunoki N, Ito T, Sakurai N, et al. A novel celecoxib derivative potently induces apoptosis of human synovial fibroblasts. J Pharmacol Exp Ther 2005; 314: 796-803.
- Tsoyi K, Park HB, Kim YM, et al. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J Agric Food Chem 2008; 56: 8969-8974.
- Tang X, Kim AL, Kopelovich L, et al. Cyclooxygenase-2 inhibitor nimesulide blocks ultraviolet B-induced photocarcinogenesis in SKH- 1 hairless mice. Photochem Photobiol 2008; 84; 522-527
- Georgakilas AG, Konsta AA, Sideris EG, et al. Dielectric and UV spectrophotometric study of physiochemical effects of ionizing radiation on mammalian macromolecular DNA. IEEE Transactions on Dielectrics and Electrical Insulation 2001; 8: 549-554.
- Koval OA, Chernolovskaya EL, Litvak VV, et al. Site_directed cleavage of DNA by an oligonucleotide conjugate with o-bromobenzoic acid in the presence of copper ions. Russian Chemical Bulletin 2003; 52: 2517-2522.
- Prasad NR, Jeyanthimala K, Ramachandran S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J Photochem Photobiol B 2009; 95: 196-203.
- Nagano S, Bush AI. Sensitive, selective, and irreversible inhibition of cyclooxygenase-2 activity by copper. Chem Med Chem 2008; 3: 223-225.
- Viossat B, Morgant G, Sorenson JR, et al. Crystallochemistry of copper (II) and zinc (II) chelates by nonsteroidal antiinflammatory drugs. Ann Pharm Fr 2002; 60: 102-114.
- Dhir A, Padi SS, Naidu PS, et al. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur J Pharmacol 2006; 535(1-3): 192-198.
- Nivsarkar M, Banerjee A, Padh H. Cyclooxygenase inhibitors: a novel direction for Alzheimer's management. Pharmacol Rep 2008; 60: 692-698.