Rapid RP HPLC method for quantitative determination of lornoxicam in tablets
- *Corresponding Author:
Date of Received: 10-03-2010
Date of Modified: 08-04-2010
Date of Accepted: 20-04-2010
Available Online: 15-05-2010
Abstract
The objective of the study was to develop a simple, rapid, specific and precise reverse phase high performance liquid chromatographic method for the determination of lornoxicam in bulk and pharmaceutical preparations. Chromato-graphic separation of the drug was performed on a eclipse C18 column (150 mm x 4.6 mm, 5 μm) as stationary phase and mobile phase used is methanol: 0.1% formic acid in water (80:20 v/v), with a flow rate of 0.8 mL min-1 and UV detection at 381 nm. The proposed method was validated for linearity, accuracy, precision, limit of detection (LOD) and limit of quantitation (LOQ). Linearity, accuracy and precision were found to be acceptable over the ranges of 0.5-20 μg/ml. It can be conveniently adopted for routine quality control analysis of lornoxicam.
Keywords
Lornoxicam, RP HPLC, validation, pharmaceutical formulation
Introduction
Lornoxicam, (6-chloro-4-hydroxy-2-methyl-N- 2-pyridinyl-2H-thieno[2,3-e]-1,2- thiazine- 3- carboxamide 1,1-dioxide, C13H10ClN3O4S2, Figure 1) is a non-steroidal anti-inflammatory drug with analgesic and antipyretic properties that belongs to the class of oxicams. It acts by nonselective inhibition of cyclo-oxygenase-1 and -2. It is prescribed for osteoarthritis, rheumatoid arthritis, acute lumbar-sciatica conditions and postoperative pain management [1].
In the literatures, a voltammetric [2], polarograhic [3], UV spectrophotmetric [4], LC/MS/MS [5-6], TLC-densitometry [7], and high performance liquid chromatographic (HPLC) [7-11] methods were reported for the analysis of lornoxicam. All the reported HPLC methods used buffer in the mobile phase and long retention time. The present study was aimed to develop a simple, rapid, precise, accurate and selective chromatographic method for the estimation of lornoxicam in bulk and dosage forms without the use of buffer in the mobile phase in short duraion.
Experimental
Chemicals and reagents
Bulk sample of lornoxicam was obtained from Hetero drugs, Hyderabad, India. The commercial samples of tablets containing 4 mg and 8 mg of lornoxicam were purchased from local market (T1 and T2). Milliq water (Millipore) water was used throughout the work. Methanol (HPLC grade) and Formic acid (HPLC grade) were procured from Sigma Aldrich (Switzerland).
Equipment
A Agilent LC system consisted of a solvent delivery system, an auto-injector fitted with 100 μL syringe, an online degasification system, and an UV/VIS photodiode array detector. The output signal was monitored and integrated using chem. station software (Agilent).
Chromatographic condition
A Agilent 1200, (Germany) HPLC instrument with a Zorbax eclipse XBD C-18 analytical column, (150 mm × 4.6 mm, 5 μm) was used for the study. The mobile phase used was methanol-water with 0.1% formic acid in water (80:20 v/v), with a flow rate of 0.8 ml/min. UV detection was made at 381 nm. The volume of injection was fixed at 20 μl. All analyses were done at ambient temperature. The mobile phase was prepared freshly and vacuumfiltered through a 0.45 μm Millipore nylon filters in the beginning of the experiment.
Preparation of standard stock solutions
The stock solution of lornoxicam (100 μg/ml) was prepared by dissolving 10 mg of Lornoxicam (99.8 %) in methanol in a standard 100 mL volumetric flask. Aliquots of 0.5 to 20 μg/ml were prepared from the stock solution.
Sample preparation
Twenty tablets were weighed and their average weight was calculated. The tablets were crushed into a homogeneous powder and a quantity equivalent to one tablet (4 mg, and 8 mg) was transferred to a 50 mL volumetric flask, dissolved in methanol and filtered through a 0.45 μm Millipore nylon filters.
Recommended procedure for standard graph
After a systematic and detailed study of various parameters involved, the following procedure and conditions are recommended for the determination of lornoxicam in pure samples and in dosage forms.
The calibration curve for lornoxicam was constructed by analyzing lornoxicam solutions containing 0.5 to 20.0 μg/ml in triplicate. The standard solutions were prepared by diluting the stock solution in mobile phase. Each of these samples (20 μl) was injected three times into the column and the peak area of absorption was determined. Standard graph was plotted by taking concentration of drug on x-axis and peak area of absorption on y-axis.
Quantification of Lornoxicam
Suitable dilutions of both the tablets were made with mobile phase so as to obtain a concentration of the drug in the range of linearity determined. Volume of sample injected into the column was 20 μl. All the determinations were made in triplicate.
Method Validation
Linearity
The standard curve was prepared in the concentration range of 0.5 to 20.0 μg/mL for lornoxicam. The linearity of these methods was evaluated by linear regression analysis, using least square analysis method.
Precision
The precision of the assay was determined in terms of repeatability (intra-day) and intermediate (interday) precision. The intra and inter-day variation in the peak area of drug solution containing 4 μg/mL of lornoxicam were calculated in terms of RSD (Relative standard deviation).
Accuracy
The accuracy of HPLC method was assessed by adding known amount of drug to a drug solution of pre-analyzed sample and subjected to the proposed HPLC method [12-13]. All solutions were prepared and analyzed in triplicate.
Limit of detection (LOD.) and limit of quantification (LOQ.)
The LOD and LOQ were determined for HPLC method. The limits were determined based on the standard deviation amongst response and slope of the curve at lowest concentrations (International Conference on Harmonization, 1997). The obtained theoretical values for LOD and LOQ were actually prepared and were cross checked by actual analysis using proposed method.
Results and Discussion
To develop a suitable and robust LC method for the determination of lornoxicam different mobile phases and columns were employed to achieve the efficient separation and resolution. The criteria employed for selecting the mobile phase for the analyses of the drugs were cost involved and time required for the analysis.
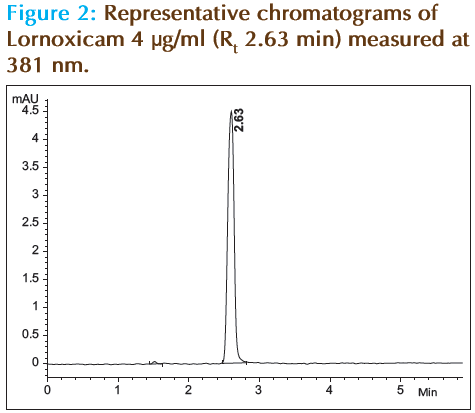
Attempts with traditional reverse phase columns presented poor peak symmetry and tailing problem. Most of the separation methods in literature overcame these problems by use of buffers in mobile phase [14]. The proposed method was able to selectively separate lornoxicam in a short chromatographic run (less than 3 min) without the use of buffer mobile phase. The retention time is 2.63 min. The chromatogram is shown in Figure 2.
System suitability
System suitability tests were performed as per the USP 31 to confirm the suitability and reproducibility of the system. The test was carried out by injecting 20 μl standard solutions of lornoxicam 10 μg/ml. This was repeated five times. The RSD values of lornoxicam were ±0.52. The RSD values were found to be satisfactory and meeting the requirements of USP 31.
Theoretical plates, tailing factor were determined and are presented in Table 1.
| Parameters | Values |
|---|---|
| Regression analysis | |
| Slope | 13.87 |
| Intercept | 0.979 |
| Correlation coefficient | 0.999 |
| Validation parameters | |
| LOD (μg/ml) | 0.013 |
| LOQ (μg/ml) | 0.416 |
| Precision (% RSD) | |
| Intra-day (n=3) | 0.99-1.52 |
| Inter-day (n=3) | 0.60-1.05 |
| Repeatability (% RSD) | 0.52 |
| System suitability test parameters | |
| Retention time (min) ± % SD | 2.63 ± 0.08 |
| Tailing factor ±% RSD | 0.786 ± 0.095 |
| Theoretical plates ±% SD | 5609.478 ± 6.5 |
Table 1: Summary of Regression analysis and validation parameters
Linearity
Linearity was evaluated by analysis of working standard solutions of lornoxicam of six different concentrations. The range of linearity was from 0.5 - 20 μg/ml. The peak area and concentration of each drug was subjected to regression analysis to calculate the calibration equations and correlation coefficients. The regression data obtained are represented in Table 1. The result shows that within the concentration range mentioned above, there was an excellent correlation between peak area and concentration of drug.
Limit of detection and limits of quantitation
The limit of detection (LOD) and limit of quantitation (LOQ) were established as per the ICH guidelines. Limit of detection and limit of quantification were found to be 0.013 μg/ml and 0.465 μg/ml of lornoxicam respectively (Table 1).
Precision
The method precision was evaluated by inter and intra-day repeatability. RSD values were found to be well below 1.52 % for intra-day repeatability and below 1.05 % for inter day repeatability indicating good precision of method (Table 1).
Accuracy
To study accuracy of the method, recovery experiment was carried out by applying the standard addition method. A known quantity of each drug substance corresponding to 50%, 100% and 150% of the label claim of each drug was added, to determine if there are positive or negative interferences from excipients present in the formulation [12-13]. Each set of addition was repeated three times. The accuracy was expressed as the percentage of analytes recovered by the assay. Table 2 lists the recoveries of the drugs from a series of spiked concentrations. The results indicate the method is highly accurate for s determination of the lornoxicam.
| Sl.No. | Std. lornoxi-cam conc.mcg/ml | Sample conc.mcg/ml | Recovery of standard Drug* mcg/ml | % Recovery of Standard*±RSD |
|---|---|---|---|---|
| 1 | 2 | 4 | 1.97 ± 0.23 | 98.50 ± 1.73 |
| 2 | 4 | 4 | 4.06 ± 0.15 | 101.50 ± 0.95 |
| 3 | 8 | 4 | 8.11 ± 0.09 | 101.37 ± 0.69 |
*Mean value of three determinations.
Table 2: Recovery of lornoxicam standard solution added to sample
| Formulation | Lornoxicam in label clam(mg) | Total amount found (mg) ± SD* | % of Lornoxicam found ±RSD* |
|---|---|---|---|
| T1 | 4 | 4.08 ± 0.19 | 102 ± 1.26 |
| T2 | 8 | 7.95 ± 0.32 | 99.37 ± 0.93 |
*Mean value of three determinations.
Table 3: Assay result for lornoxicam (4 mg and 8 mg per tablet) in the formulation product
Specificity
Specificity is the ability of the method to accurately measure the analyte response in the presence of all potential sample components (excipients). The results were compared with the analysis of a standard lornoxicam and tablet formulations. Excipients of the solid dosage form did not interfere with the analyte.
Conclusion
The proposed method for quantitative determination of lornoxicam in pharmaceutical formulation is efficient and sensitive. The excipients of the commercial sample analyzed did not interfere in the analysis, which proved the specificity of the method for these formulations. The HPLC method was found to be simple, rapid, precise, accurate and sensitive. Its advantages over other existing methods are its low-cost, non usage of buffers and less time consuming. This method can be used for routine quality control of lornoxicam in commercial samples.
References
- Balfour JA, Fitton A and Barradell LB, Lornoxicam. A review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions. Drugs. 1996; 51(4): 639-657.
- Ghoneim MM, Beltagi AM and Radi A, Square-wave Adsorptive Stripping Voltammetric Determination of the Anti-inflammatory Drug Lornoxicam. Anal. Sci. 2002; 18(2): 183-186.
- Ibrahim Çetin, Nisa Koçak, Sule Aycan, Polarographic determination of lornoxicam in pharmaceutical formulations. C.B.U. J. Sci. 2009; 5(1): 11 – 18.
- Nemutlu E, Demircan S and Kir S, Determination of lornoxicam in pharmaceutical preparations by zero and first order derivative UV spectrophotometric methods. Pharmazie. 2005; 60(6): 421-425.
- Young Hoon Kim, Hye Young Ji, Eun -Seok Park, et al. Liquid chromatography- electrospray lonization tandem mass spectrometric determination of lornoxicam in human plasma. Arch Pharmacal Res. 2007 July; 30(7): 905-910.
- Zeng YL, Chen XY, Zhang YF, et al. Determination of lornoxicam in human plasma by LC/MS/MS. Yao Xue Xue Bao. 2004 Feb;39(2):132-5.
- Nagiba Y. Hasan, Mohamed A. Elkawy, Badr E. Elzeany, et al. Stability indicating methods for the determination of lornoxicam, from http://pharma.cu.edu.eg/English/PostgradAffair/Publications/Journal/Volums/TOC_2004_V42_Issue_3/001.htm.
- Radhofer-Welte S and Dittrich P. Determination of the novel nonsteroidal anti-inflammatory drug lornoxicam and its main metabolite in plasma and synovial fluid. J. Chromatogr. B. 1998; 707(1-2): 151-159.
- Zhang JJ, Gao Y, Fan WM, et al. Development and validation of a stability-indicating HPLC method for the estimation of lornoxicam in pharmaceutical formulation, from http://www.aapsj.org/abstracts/AM_2004/AAPS2004-000063.PDF.
- Akiko Nakamura, Nakashima MN, Mitsuhiro Wada et al. Semi-Micro Column HPLC of Three Oxicam Non-Steroidal Anti-Inflammatory Drugs in Human Blood. Bunseki Kagaku. 2005;54: 755.
- Taha EA, Salama NN, Abdel Fattah Lel-S. Stability-indicating chromatographic methods for the determination of some oxicams. J AOAC Int. 2004 Mar-Apr;87(2):366-73.
- Snyder LR, Kirland JJ and Glajch JL, Practical HPLC method development, 2nd Ed., John Wiley and Sons, Inc., U.S.A, 1997.
- ICH, Validation of analytical procedures: methodology, ICH harmonized tripartite guidelines, Published in the Federal Register. 1997; 62(96): 27463-27467.
- Barbara OM, Malgorzata J and Ludomir K. The effect of stationary phase type and mobile phase pH on the separation of some catecholamines. J. Pla Chroma. 2001;14(4): 256-259.