Protective effects of curcumin, vitamin C, or their combination on cadmium-induced hepatotoxicity
- *Corresponding Author:
Date of Received: 27-04-2012
Date of Accepted: 04-05-2012
Available Online: 15-05-2012
Abstract
Curcumin, a biologically active compound from turmeric, and vitamin C act as a natural antioxidant and potent chemopreventive agent. The objective of the study was to investigate whether the combined pretreatment with curcumin and vitamin C offers more beneficial effects than that provided by either of them alone in reversing cadmium (Cd)- induced hepatotoxicity. For this purpose, 64 adult male Wistar rats, equally divided into control and seven treated groups, received either Cd (as CdCl2 5 mg/kg), curcumin 400 mg/kg, curcumin 200 or 400 mg/kg + CdCl2, vitamin C 100 mg/kg + CdCl2, curcumin 200 or 400 mg/kg + vitamin C + CdCl2. All groups were treated by gavage for 27 days. The results showed that Cd treatment increased significantly lipid peroxidation levels,decreased significantly the glutathione levels, increased significantly on metallothionein (MT) expressions including the degenerative changes of liver histological tissues were observed. The treatment of Cd-exposed rats with curcumin or vitamin C alone could not reverse Cd-induced the above changes. The combined treatment with curcumin along with vitamin C before Cd intoxication was more effective than that with either of them alone in reducing such changes and reverse the changes almost similar to that of control. In conclusion, the results demonstrated that the combined pretreatment with curcumin along with vitamin C could recover the alterations and offer more protection than curcumin or vitamin C alone against Cd hepatotoxicity.
Keywords
curcumin, vitamin C, cadmium toxicity, liver, metallothionein
Introduction
Cadmium (Cd) is a widespread environmental pollutant, hav- ing diverse toxicity in various organs of man and animals and is classified as a human carcinogen [1]. Cd contamination of envi- ronment is a subject of serious international concern since the metal is known to enter the food chain and can undergo bioaccumulation, end angering human health. The liver makes up the bulk of total body burden to Cd because of its ability to produce large amount of metallothionein, a metal-binding protein with high affinity for Cd [2]. The mechanism responsible for Cd toxicity may be multifactorial. Cd can cause oxidative damage within tissues, which is considered an early sign of toxicity and has been linked with carcinogenesis [3].
Cd exerts its toxic effects via oxidative damage to cellular organelles by inducing the generation of reactive oxygen species (ROS) [4] which consist mainly of •O2 , H2O2 and •OH. The molecular mechanism by which the generation of free radicals are far from being understood but reports have indicated that Cd does this via an indirect phenomenon [5]. In addi- tion to that depletion of glutathione and other endogenous antioxidants may also contribute significantly to the development of Cd-induced toxic oxidative stress [6]. If these ROS-mediated stress events are not balanced by repair processes, affected cells undergo apoptosis or necrosis [7].
Antioxidants are the natural defense mechanism existing in our system and these are capable of scavenging the deleterious free radicals. A number of dietary antioxidant compounds have been shown to influence the membrane characteristics such as fluidity, stability and susceptibility to membrane oxidative damage [8]. Recently, a great deal of attention has focused on the protective biochemical functions of naturally occur- ring antioxidants in biological systems against toxic heavy metals. Thus it is believed that antioxidant should be one of the important compo- nents of an effective treatment of Cd poisoning. Curcumin (diferuloyl- methane), a yellow coloring ingredient of the spice turmeric obtained from the rhizomes of Curcuma longa Linn (Zingiberacea), a perennial herb distributed mainly throughout tropical and subtropical regions of the world. Curcumin represents a class of anti- inflammatory and anti- oxidants reported to be a potent inhibitor of ROS formation [9, 10]. Reddy and Lokesh [11] indicated that curcumin is a potent scavenger of a variety of ROS including superoxide anion radicals and hydroxyl radicals. Curcumin administration has been reported to prevent the arsenic, gentamicin and acetaminophen-induced oxidative stress in rats [12, 13, 14]. The protective effects of curcumin against chemically-induced hepa- totoxicity are well documented, and have been attributed to its intrinsic antioxidant properties [15, 16]. In addition, vitamin C or ascorbic acid exhibit a protective effect against free radical-induced oxidative damage [17]. Its scavenging effect of oxygen radicals has been clarified. It can participate in the redox mechanism of the cell, and thereby neutralize ROS.
There are multitudes of reports available on the protective effects of curcumin, vitamin C individually against various xenobiotics induced oxidative stress in experimental animals. Still to date the reports are scanty regarding the combined alleviated efficacy of curcumin in combi- nation with vitamin C on Cd induced hepatotoxicity in rats. As well as there are some controversies over the combined administration of curcu- min in combination with vitamin C. In view of the above considerations, the present study was designed to evaluate the protective efficacy of cur- cumin in combination with vitamin C on Cd induced oxidative damages in the liver of rats.
Materials and Methods
Chemicals
Curcumin was purchased from Cayman Chemical Company. CdCl2, Vitamin C, Bovine serum albumin and Bradford reagent was purchased from Sigma-Aldrich Chemical Company, St. Louis, USA. The other chemicals used, eg. absolute ethanol was purchased from Merck (Darm- stadt, Germany) and all the reagents were of analytical grade.
Animals
Adult male Wistar rats were used in the present study. The experimen- tal animals were supplied by the National Laboratory Animal Center of Mahidol University and used for experiments after 1 week of acclima- tization. The animals were maintained as national guidelines and pro- tocols, approved by the Institutional Animal Ethics Committee and in an air-conditioned animal house with constant 12 h light and 12 h dark schedule. Animals were fed on standardized diet for rodents and water adlibitum.
Experimental design
Curcumin, vitamin C and CdCl2 was dissolved in sterile distilled water. The dose of CdCl2, curcumin and vitamin C used in this study was se- lected on the basis of the previous study [18, 19, 20]. Curcumin or vita- min C was given daily by oral gavage for 1 h before CdCl2 administration. The experiment was conducted over a period of 27 days. The animals, at 200-220 g initial body weight, were randomly divided into eight groups of 8 animals each.
Group 1: Control rats received only distilled water for a period of 27 days.
Group 2: Normal rats orally received curcumin (400 mg/kg BW) alone for a period of 27 days.
Group 3: Normal rats orally received cadmium chloride (5 mg/kg BW) for 27 days.
Group 4: Normal rats orally received curcumin (200 mg/kg BW) for 1 h prior to Cd (5 mg/kg BW) for 27 days.
Group 5: Normal rats orally received curcumin (400 mg/kg BW) for 1 h prior to Cd (5 mg/kg BW) for 27 days.
Group 6: Normal rats orally received vitamin C (100 mg/kg BW) for 1 h prior to Cd (5 mg/kg BW) for 27 days.
Group 7: Normal rats orally received curcumin (200 mg/kg BW) in combina- tion with vitamin C (100 mg/kg BW) for 1 h prior to Cd (5 mg/kg BW) for 27 days.
Group 8: Normal rats orally received curcumin (400 mg/kg BW) in combina- tion with vitamin C (100 mg/kg BW) for 1 h prior to Cd (5 mg/kg BW) for 27 days.
All groups of rats were treated by oral gavage once daily for 27 days. All the animals were sacrificed 24 h after the last treatment following protocols and ethical procedures.
The liver was immediately dissected out, weighed and washed using chilled saline solution. Tissues were minced and homogenized (10%w/v), separately, in ice-cold 0.1 M phosphate buffer (pH 7.4) using glass homogenizer. The homogenate was used for the determina- tion of MDA and reduced GSH. The remaining liver was fixed in 10% neutral phosphate buffer formalin solution for histological analysis and immunohistochemistry of metallothionein.
Malondialdehyde (MDA) and reduced glutathione (GSH) assays
Lipid peroxidation (LPO) level was measured by the method of Buege and Aust [21] and evaluated by measuring the malondialdehyde (MDA) concentration which was the end product of LPO. The tissues homoge- nates were precipitated with trichloroacetic acid. After centrifugation (1500×g, 15 min), the supernatant was mixed with thiobarbituric acid (TBA) reagent and the mixture was kept at 100 °C for 15 min. The level of LPO was measured based on the formation of TBA reactive substance (TBARS) to produce a red colored complex with a peak absorbance at 535 nm. An extinction coefficient of 1.56 X 105 M-1 cm-1 was applied for calculation and results were expressed as nM/mg protein.
Reduced GSH was determined according to the method by Beutler [22] using Ellman’s reagent. The procedure is based on the reduction of Ellman’s reagent by SH groups to produce 5,5’-dithiobis (2-nitrobenzoic acid) which has an intense yellow color that is measured spectrophoto- metrically at 412 nm using Thermo Scientific Genesis 10S spectropho- tometer. GSH levels were calculated using an extinction coefficient of 1.36 × 105 M-1cm-1. Results were expressed as μM/mg protein.
The protein content in supernatant was estimated by the method of Bradford et al. [23] using bovine serum albumin (BSA) as the standard.
Histopathological studies
Immediately after sacrifice, the liver was removed surgically and rinsed with ice cold physiological saline. For microscopic evaluation liver was fixed in 10% neutral phosphate buffer formalin solution for 48 h. Follow- ing dehydration in ascending series of ethanol (70, 80, 95, 100%), tissue samples were cleared in xylene and embedded in paraffin. Tissue sections of 5.0 μm were stained with hematoxylin and eosin (H&E). These sec- tions were examined under light microscopy (Axioskop 40, Zeiss) and documented by digital photocamera (Axiocam, Zeiss).
Immunohistochemistry of Metallothionein
For immunohistochemical detection of MT protein, the sections of liver tissues on poly-L-lysine coated slides were deparaffinized in xylene and hydrated in ethanol series. Antigen retrieval was performed with 10 mM citrate buffer, pH 6 for 15 min. After incubation with 10% normal goat serum for 5 min at room temperature, sections were incubated overnight at 4 °C with the primary antibody mouse anti-metallothionein (Invit- rogen, clone E9) in 1% BSA using 1:50 dilution. To detect the specific binding of the primary antibody, an immunohistochemical staining kit (Histostatin Plus, Zymed laboratories, USA) was used, with which tis- sues were incubated sequentially with a biotinylated secondary antibody for 2 h at room temperature. This was followed by streptavidin peroxidase complex. Finally, diaminobenzidine was used as chromogen. Sections were counter stained with hematoxylin. Preparations were evaluated by a bright field microscope and were photographed (Axioskop 40, Zeiss). Negative control sections were prepared by substituting the primary an- tibodies with phosphate-buffer saline.
MT immunostaining was considered positive when the nuclei and cytoplasm of the hepatocytes were stained prominently (purplish brown or reddish brown). The percentage of cells with immunostained MT was determined by an automated image analyzer (Axiocam, Zeiss) after total and MT-stain-positive cells were counted at 100× magnification in three random fields (90 nm2) of three different animals. Results were expressed as percentage of positive cells.
Statistical Analysis
All the data were expressed as mean±SEM (standard error of the mean). One-way analysis of variance (ANOVA) followed by a post hoc test was carried out to test for any differences between the mean values of all groups. A p-value of <0.05 and 0.01 was considered statistically signifi- cant.
Results
Effects of curcumin and vitamin C on MDA and reduced GSH levels in rat liver induced by cadmium
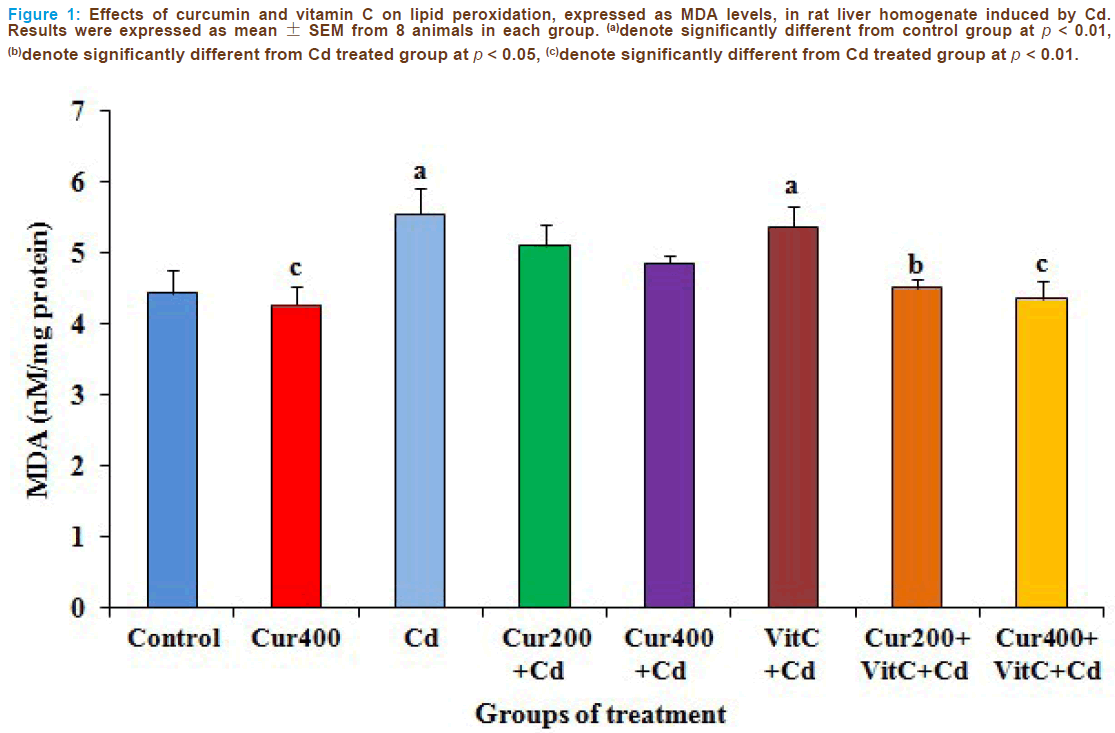
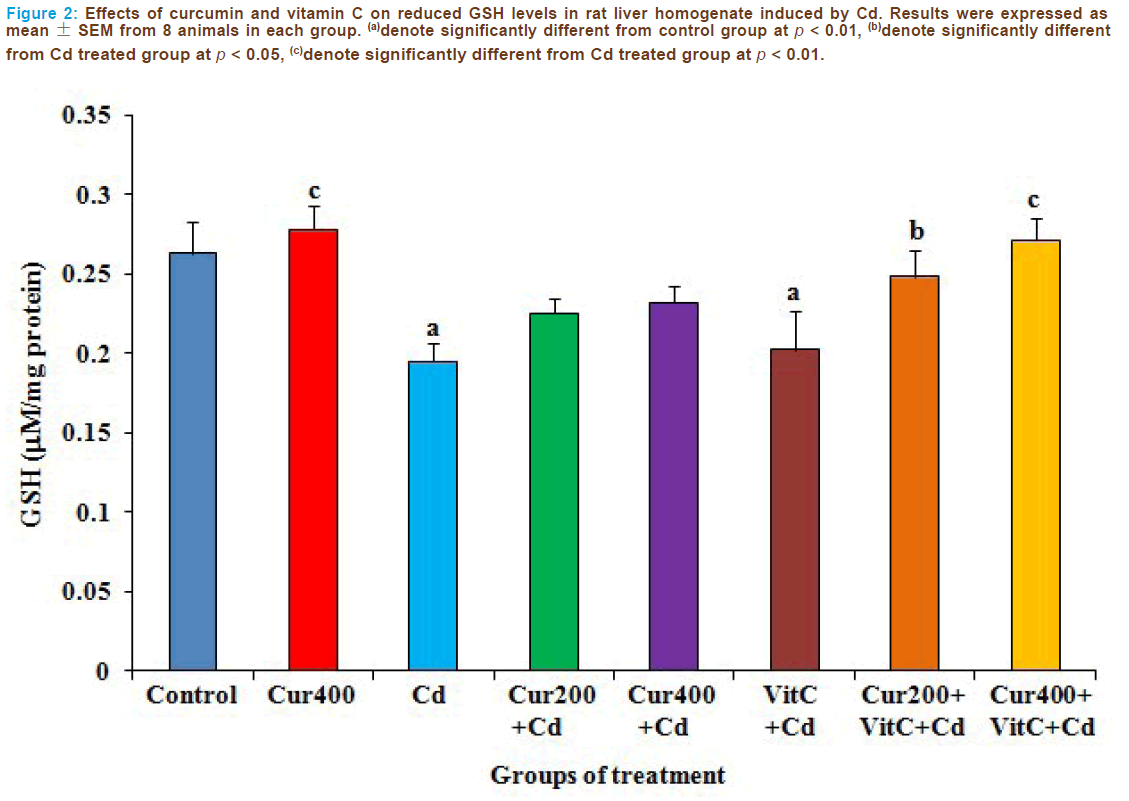
MDA levels in the liver tissue were used as a measure of lipid peroxida- tion. Figures 1 and 2 showed the changes of MDA and reduced GSH levels respectively in all groups. The MDA and reduced GSH levels were similar in the control and curcumin 400 mg/kg groups (p>0.05). Administration of Cd caused significantly increase of MDA levels and significantly decrease of reduced GSH levels as compared to the con- trol group (p <0.01). The administrations of curcumin and vitamin C alone before Cd intoxication could not reverse the changes of MDA and reduced GSH levels as compared to the Cd treated group. The above changes were reversed in the combined pretreatment with curcumin 200 (p < 0.05) or 400 mg/kg (p < 0.01) and vitamin C when compared to the Cd treated group. Therefore, the combined pretreatment with curcumin particularly at the dose of 400 mg/kg and vitamin C could attenuate Cd intoxication in rat liver.
Figure 1: Effects of curcumin and vitamin C on lipid peroxidation, expressed as MDA levels, in rat liver homogenate induced by Cd. Results were expressed as mean ± SEM from 8 animals in each group. (a)denote significantly different from control group at p < 0.01, (b)denote significantly different from Cd treated group at p < 0.05, (c)denote significantly different from Cd treated group at p < 0.01.
Figure 2: Effects of curcumin and vitamin C on reduced GSH levels in rat liver homogenate induced by Cd. Results were expressed as mean ± SEM from 8 animals in each group. (a)denote significantly different from control group at p < 0.01, (b)denote significantly different from Cd treated group at p < 0.05, (c)denote significantly different from Cd treated group at p < 0.01.
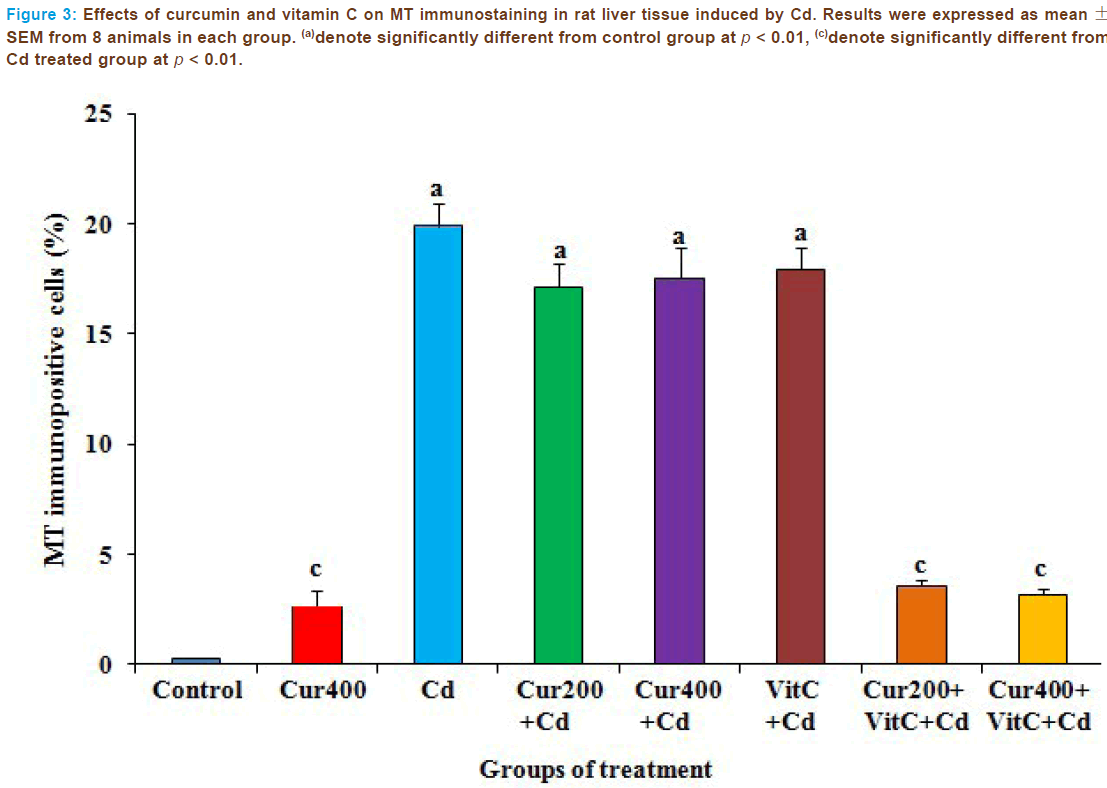
Figure 3: Effects of curcumin and vitamin C on MT immunostaining in rat liver tissue induced by Cd. Results were expressed as mean ± SEM from 8 animals in each group. (a)denote significantly different from control group at p < 0.01, (c)denote significantly different from Cd treated group at p < 0.01.
Effects of curcumin and vitamin C on immunohistochemis- try of metallothionein and histological analysis in rat liver induced by cadmium
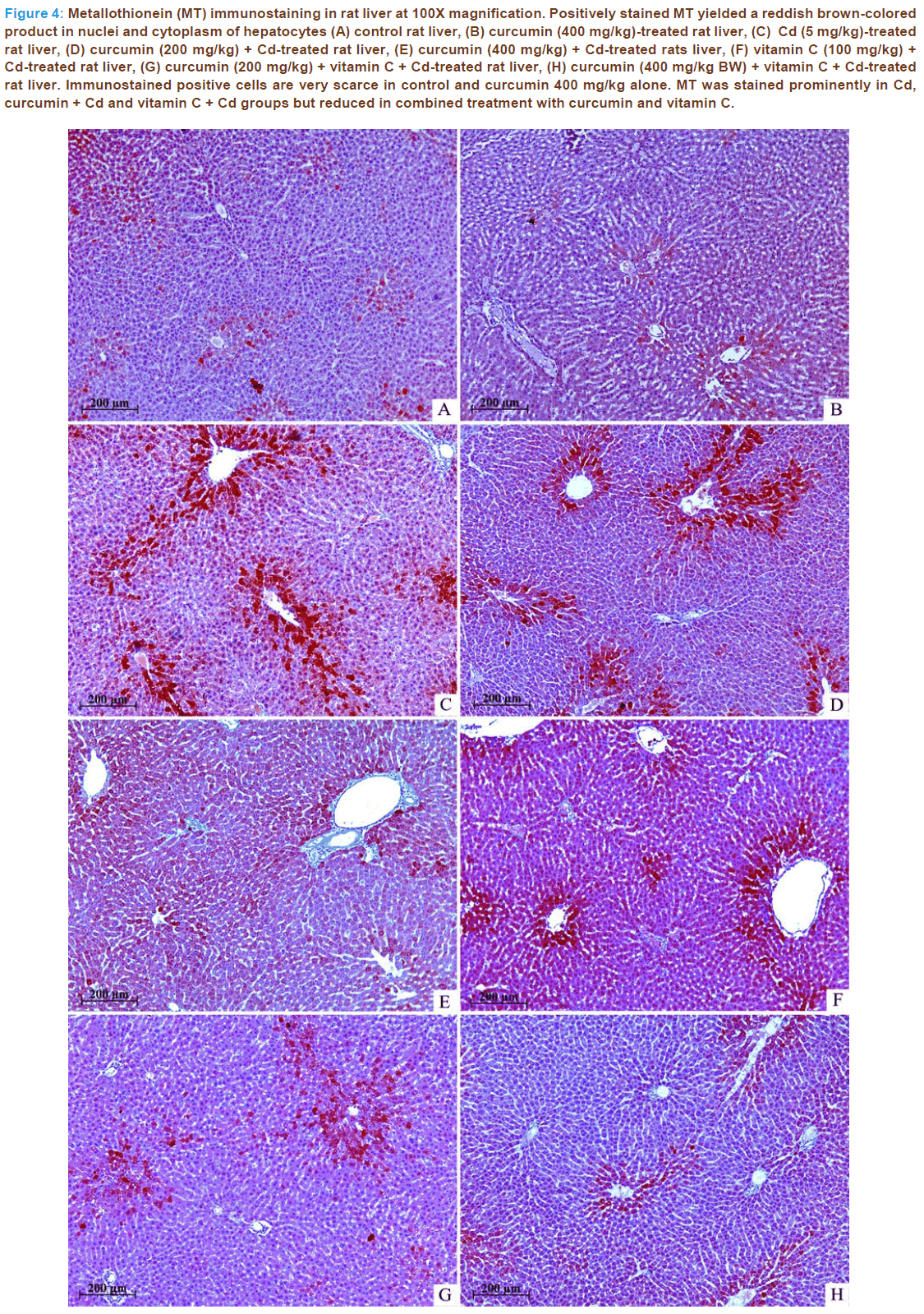
MT immunostaining in all groups were shown in Figure 4. Immunos- tained positive cells are very scarce in control and curcumin 400 mg/kg alone. In liver of Cd-treated rats, MT expressions were significantly in- creased (p < 0.01) as compared to the control group. The administrations of curcumin and vitamin C alone before Cd intoxication could not reduce such changes when compared to the Cd-treated group. The combined treatment with curcumin 200 or 400 mg/kg and vitamin C before Cd intoxication reduced significantly (p < 0.01) on MT expressions as com- pared to the Cd-treated group and reverse the changes almost similar to that of control.
Figure 4: Metallothionein (MT) immunostaining in rat liver at 100X magnification. Positively stained MT yielded a reddish brown-colored product in nuclei and cytoplasm of hepatocytes (A) control rat liver, (B) curcumin (400 mg/kg)-treated rat liver, (C) Cd (5 mg/kg)-treated rat liver, (D) curcumin (200 mg/kg) + Cd-treated rat liver, (E) curcumin (400 mg/kg) + Cd-treated rats liver, (F) vitamin C (100 mg/kg) + Cd-treated rat liver, (G) curcumin (200 mg/kg) + vitamin C + Cd-treated rat liver, (H) curcumin (400 mg/kg BW) + vitamin C + Cd-treated rat liver. Immunostained positive cells are very scarce in control and curcumin 400 mg/kg alone. MT was stained prominently in Cd, curcumin + Cd and vitamin C + Cd groups but reduced in combined treatment with curcumin and vitamin C.
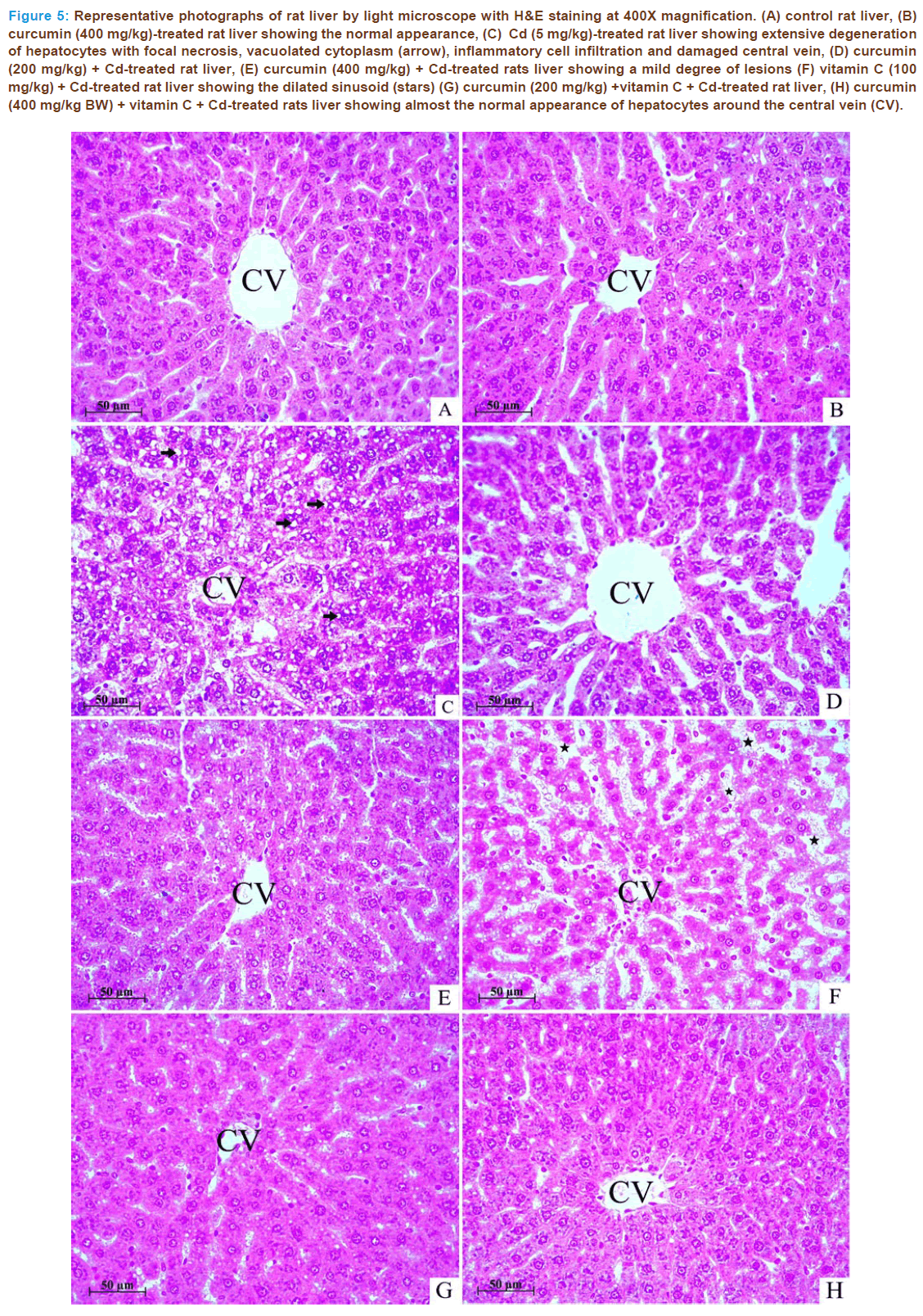
Figure 5: Representative photographs of rat liver by light microscope with H&E staining at 400X magnification. (A) control rat liver, (B) curcumin (400 mg/kg)-treated rat liver showing the normal appearance, (C) Cd (5 mg/kg)-treated rat liver showing extensive degeneration of hepatocytes with focal necrosis, vacuolated cytoplasm (arrow), inflammatory cell infiltration and damaged central vein, (D) curcumin (200 mg/kg) + Cd-treated rat liver, (E) curcumin (400 mg/kg) + Cd-treated rats liver showing a mild degree of lesions (F) vitamin C (100 mg/kg) + Cd-treated rat liver showing the dilated sinusoid (stars) (G) curcumin (200 mg/kg) +vitamin C + Cd-treated rat liver, (H) curcumin (400 mg/kg BW) + vitamin C + Cd-treated rats liver showing almost the normal appearance of hepatocytes around the central vein (CV).
The histological analysis of liver revealed that control (Fig. 5A) and curcumin-alone-treated rats (Fig. 5B) showed normal histoarchitecture. The administration of Cd in rats produced severe hepatic damage includ- ing the extensive degeneration of hepatocytes with necrosis, inflamma- tion, cytoplasmic vacuolization and inflammatory cell infiltration (Figs. 5C) when compared to control rats (Fig. 5A). The cellular infiltrations and vacuolization were localized around the central vein.The administra- tions of curcumin and vitamin C alone before Cd intoxication showed a mild degree of lesions (Fig. 5D, 5E and 5F) when compared to the Cd- treated group. The combined treatment with curcumin 200 or 400 mg/ kg and vitamin C before Cd intoxication reduced such changes and kept the organ almost similar to that of control (Fig. 5G and 5H). Results of the histological assessment support the outcome of the earlier studies by exhibiting Cd-induced necrosis in the liver tissue and its protection by combined pretreatment with curcumin and vitamin C.
Discussion
In the present study, we found that subacute Cd intoxication induced liver damages, measured by increased lipid peroxidation and decreased reduced GSH. The damages were also associated with histopathologi- cal changes and immunohistochemistry of MT staining. The purpose of the present study is to evaluate the potential benefit of curcumin in combination with vitamin C on Cd-induced hepatotoxicity compared to curcumin or vitamin C treatment alone in rats. Several lines of stud- ies showed that curcumin prevent the arsenic, gentamicin and acetami- nophen-induced oxidative stress in rats [12, 13, 14]. Vitamin C might also have a structural role in stabilizing membranes [24]. The present study has shown that administration of curcumin in combination with vitamin C significantly reversed the levels of MDA, reduced GSH, MT expressions and histopathological changes of liver tissues, which in- fers that the Cd induced alterations in the liver membrane might have been protected through membrane stabilization effects of curcumin and vitamin C.
Lipid peroxidation is one of the main manifestations of oxidative damage and has been found to play an important role in the toxicity of Cd.Cd induced oxidative stress by producing hydroxyl radicals, superox- ide anions, nitric oxide and hydrogen peroxide [25]. Significant increase in the level of hepatic TBARS in Cd intoxicated rats could be possibly due to excessive formation of free radicals which leads to the deteriora- tion of biological macromolecules [26]. Milton Prabu et al. [27] have reported that lipid peroxidation is considered a sensitive marker of Cd hepatotoxicity. Manca [28] have also reported the increased level of LPO in the various tissues of Cd treated rats. In the present study, we observed a marked elevation of hepatic LPO following Cd administration was in consistence with the other reports in Cd- intoxicated rats [29].
The impairment of the antioxidant defense system is considered as a critical event in Cd-induced hepatotoxicity. Exposure of Cd is char- acterized by the depletion of tissue and circulating non-enzymatic anti- oxidants including GSH [30]. GSH is a sulfhydryl peptide enormously present in all biological systems and participates in the maintenance of cytoplasmic and membrane thiol status. It forms the first line of defense against oxidative insult by acting as a non-enzymatic antioxidant by direct interaction of its sulfhydryl group with ROS or it can be involved in the enzymatic detoxification reaction of ROS as a cofactor or as a coenzyme. Cd binds exclusively to sulfhydryl groups of GSH leading to its inactivation [31]. In the present study, the reduced level of hepatic GSH by Cd could be probably due to either increased utilization of GSH by the cells act as scavengers of free radicals produced by Cd or increased utilization of GSH for the activity of GPx forming oxidized GSH (GSSG) due to increased generation of ROS [32]. Our findings are in consonance with the other published reports which quoted that GSH concentration is decreased during Cd intoxication [33].
Histopathological results also supported the biochemical findings. According the light microscopic examination, it was clearly demon- strated that Cd administration caused a significant abnormality of liver morphology (Fig. 5), showing the extensive degeneration of hepato- cytes with necrosis, inflammation, vacuolization, inflammatory cell in- filtration and fatty degenerative changes (panel C), as compared with control (panel A). Treatment with curcumin and vitamin C (panel G and H) before Cd administration resulted in the improvement of liver cell damages observed with curcumin or vitamin C alone, compare panel E and F with panel G and H. This histopathological analysis is in agreement with the observed result in the MDA, a biochemical indicator of necrosis. Similar changes in the hepatic tissue of Wis- tar rats have been reported by previous findings of Milton et al. [34]. These changes could be the results of membrane distribution induced by Cd. In fact, this metal promotes an early oxidative stress. After- wards it contributes to the development of various pathological aspects in soft tissues including liver. The current study obviously shows that histopathologic changes in liver are associated with the increasing of LPO and reducing GSH in conjunction by previous reports [35]. Ad- ministration of curcumin in combination with vitamins C notably re- duced the histological alterations evoked by Cd quite appreciably. It can be attributed to their antilipoperoxidative, antioxidant, and metal chelating properties, which significantly reduced the oxidative threat leading to reduction of pathological changes and restoration of its nor- mal physiological function.
Metallothioneins (MT) are a group of low molecular weight cystein- rich important antioxidant protein in the cellular defence against Cd toxicity. They have the ability to bind and sequestrate Cd. It is well es- tablished that Cd significantly enhances hepatic MT gene expression and MT synthesis [36]. In the present study, MT was stained prominently in hepatocytes of Cd-treated group might be related with accumulation side of this metal to prevent the free Cd ions from exerting their toxic effects. The results of MT immunostaining confirm the biochemical and his- topathological changes. This indicated that Cd caused the hepatotoxicity by increasing of MT expressions and treatment with curcumin or vitamin C alone could not protect the hepatotoxicity induced by Cd.
Cellular damages caused by Cd exposure can be prevented by free radical scavengers or antioxidants, which further strengthens the hypothesis that free radicals play a key role in Cd toxicity. Curcumin, the most abundant curcuminoid compound in turmeric (C. longa), has multifunctional actions. The antioxidant mechanism of curcumin is due to its specific conjugated structure of two methoxylated phenols and an enol form of β-diketone. This structure is responsible for free radical trapping ability as a chain breaking antioxidant [37]. The abil- ity of curcumin to chelate the toxic metals was shown by Daniel et al. [38]. They found that curcumin significantly protects against lipid peroxidation induced by heavy metals, lead and cadmium in the rat brain homogenate, as well as reduces lead-induced structural damage in the hippocampus. Curcumin has been reported to attenuate liver injury induced by diverse hepatotoxicants [39, 40, 41] via multiple mechanisms. The two doses of curcumin selected in this study were based on previous studies in which curcumin showed protective effect against oxidant damage in the heart [42], liver [43], and kidney [44]. Its efficacy in protecting against liver injury has been established by its antioxidant or free radical-scavenger action. In our study, we found that the treatment of Cd-exposed animals with curcumin (400 mg/ kg) alone partially reversed Cd-induced increase in MDA and Cd- induced decrease in reduced GSH although no significantly effect. This result is supported by our previous study shown that curcumin partially protect against Cd-induced nephrotoxicity [45]. However, the treatment had done on shorter period (5 days) and lower doses of curcumin (250 mg/kg) used in the previous study.
Vitamin C (ascorbic acid) is an important dietary antioxidant, it significantly decreases the adverse effect of reactive species such as reactive oxygen and nitrogen species that can cause oxidative damage to macromolecules such as lipids, DNA and proteins which are implicated in chronic diseases including cardiovascular disease, stroke, cancer, neu- rodegenerative diseases and cataractogenesis. Ascorbic acid is a potent water soluble antioxidant capable of scavenging/neutralizing an array of reactive oxygen species viz., hydroxyl, alkoxyl, peroxyl, superoxide anion, hydroperoxyl radicals and reactive nitrogen radicals such as nitrogen di- oxide, nitroxide, peroxynitrite at very low concentrations [46]. Vitamin C is an essential cofactor for many enzymes involved in diverse metabolic pathways [47]. Sahin et al. [48] found that serum activities of SGOT and SGPT were not influenced by dietary vitamin C. Also, Yousef et al. [49] and Yousef [50] found that rabbits treated with vitamin C did not show any changes in the activities of AST and ALT. This is in accordance with our results showing that vitamin C had no significant effects in preven- tion against liver damages in Cd intoxicated rats.
Conclusion
Therefore, it is new findings in the present study which is different from the previous study. The results of the present study demonstrated that the curcumin in combination with vitamin C had a significant hepatopro- tective action on Cd induced oxidative damage in the liver tissue of rat. Cd decreased the GSH level and disturbed the redox state of the cells. The observed data infers that increased lipid peroxidation and associated ROS generation, decline in antioxidant status might have culminated in collapse of membrane integrity that lead to liver damage in Cd intoxicated rats. Reversal of these abnormalities to a near normal status reflects the causal association of antioxidant/anti-radical properties of curcumin and vitamin C. Further the hepatic protection was maximum in the combined treatment of curcumin in combination with vitamin C than the curcumin or vitamin C alone in the Cd intoxicated rats. Curcumin and vitamin C might show their protective effects as a scavenger of free radicals. This feature might also contribute to their antioxidant activity. Nevertheless, this feature needs to be further investigated.
In conclusion, our findings indicate treatment of combined curcumin and vitamin C inhibits remarkably liver damages in Cd intoxicated rats. Combination form of antioxidants might be very useful in protection of liver against Cd toxicity.
Acknowledgement
The authors are thankful to Rangsit University and National Research Council of Thailand (NRCT) for supporting and funding this project.
References
- IARC. Meeting of the IARC working group on beryllium, cadmium, mercury and exposures in the glass manufacturing industry. Scand J Work Environ Health.1993; 19: 360–363.
- Waisberg M, Joseph P, Hale B, et al. Molecular mechanisms of cadmium carcinogenesis. Toxicol. 2003; 192: 95–117.
- Galaris D, Evangelou A. The role of oxidative stress in mechanisms of metal induced carcinogenesis. Crit Rev Oncol Hematol. 2002; 42: 93–103.
- Stohs SJ, Bagchi D, Hassoun E, et al. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2000;20:77–88.
- Watkin RD, Nawrot T, Potts RJ, et al. Mechanisms regulating the cadmium mediated suppression of Spl transcription factor activity in alveolar epithelial cells. Toxicol. 2003;18:157–178.
- Bagchi D, Bagchi M, Hassoun EA, et al. Cadmium induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion and hepatic lipid peroxi-dation in Sprague-Dawley rats. Biol Trace Elem Res. 1996; 52: 143–154.
- Thevenod F. Nephrotoxicity and the proximal tubule insights from cadmium. Nephron Physiol. 2003; 93: 87–93.
- Halliwell B, Gutteridge JMC. Role of free-radicals and catalytic metal-ions in human-disease-an overview. Method Enzymol. 1990;186:1–85.
- Venkatesan N, Punithavathi D, Arumugan V. Curcumin prevents adriamycin nephrotoxicity in rats. Br J Pharmacol. 2000; 12: 231–234.
- Biswas SK, McClure D, Jimenez LA, et al. Curcumin induces glutathione biosynthesis and inhibits NFkappaB activation and interleukin-8 release in al-veolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005; 7: 32–41.
- Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of fer-rous ion. Mol Cell Biochem. 1994;137:1–8.
- Fatma MED, Mokhtar IY, Fatma MER. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009; 47: 249–254.
- Farombi EO, Ekor M. Curcumin attenuates gentamicin-induced renal oxida-tive damage in rats. Food Chem Toxicol. 2006; 44: 1443–1448.
- Cekmen M, Ilbet YO, Ozbek E, et al. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 2009; 47: 1480–1484.
- Nanji AA, Jokelainen K, Tipoe GL, et al. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003; 284: G321–G327.
- Rukkumani R, Aruna K, Varma PS, et al. Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci. 2004; 7: 274–283.
- Padayatty S, Sebastian J, Katz A, et al. Vitamin C as an antioxidant: evalua-tion of its role in disease prevention.J Am Coll Nutr. 2003; 22: 18–35.
- Chuang SE, Chen AL, Lin JK, et al. Inhibition by curcumin of diethylnitro-samine- induced hepatic hyperplasia, inflammation, cellular gene product and cell-cycle- related protein in rats. Food Chem Toxicol. 2000;38:991–995.
- Milton Prabu S, Shagirtha K, Renugadevi J.Quercetin in combination with vitamins (C and E) improve oxidative stress and hepatic injury in cadmium intoxicated rats. Biomed Prevent Nutrit. 2011; 1: 1–7.
- EI-demerdash FM, Yousef IM, Kedwany FS, et al. Cadmium induced changes in lipid peroxidation, blood heamatology, biochemical parameters and serum quality of male rats: protective role of vitamin E and β-carotene. Food Chem Toxicol.2004; 42: 1563–1571.
- Buege JA, Aust SD. Microsomal lipid peroxidation. In: Methods Enzymology. Colowick SP, Kaplan NO, ed. New York: Academic Press;1978; 52: 302-310.
- Beutler E. Glutathione in red blood cell metabolism. In: A Manual of Bio-chemical Methods. 2nd ed.New York:Grune & Stratton; 1975;112:69.
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bio-chem.1978; 72: 248–254.
- Young IS, Woodside JV.Antioxidants in health and disease. J Clin Pathol. 2001; 54: 176–186.
- Weisberg M, Joseph P, Hale B, et al. Molecular and cellular mechanisms of cadmium carcinogenesis: a review. Toxicol. 2003; 192: 95–117.
- Stohs SJ, Bagchi D, Hassoun E, et al. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2000; 19: 201–213.
- Milton Prabu S, Renugadevi J, Ramesh Kumar T. Ameliorative effect of se-lenium against cadmium induced biochemical alterations in Cirrhinus mrigala (Hamilton). Asian J Biosci.2007; 2: 143–148.
- Manca D. In vitro and in vivo response of rat tissue to cadmium induced lipid peroxidation. Bull Environ Contamin Toxicol.1991; 46: 929–936.
- Santos FW, Zeni G, Favero AM, et al. Diphenyl diselenide reverse cadmium in-duced oxidative damage on mice tissues. Chem Biol Interact. 2005; 151: 159–165.
- Hossain SK, Bhattacharya S. Prevention of cadmium induced lipid peroxida-tion, depletion of some antioxidative enzymes and glutathione by a series of novel organoselenocyanates. Environ Toxicol Pharmacol. 2006; 22: 298–308.
- Sunitha S, Nagaraj M, Varalakshmi P. Hepatoprotective effect of lupeol and lupeol linoleate on tissue antioxidant defense system in cadmium induced hepatotoxicity in rats. Fitoterapia. 2001; 72: 516–523.
- Larson RA. The antioxidants of higher plants. Phytochem. 1988; 27: 969– 978.
- Pari L, Murugavel P. Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol. 2005; 20: 493–500.
- Milton Prabu S, Muthumani M, Shagirtha K. Protective effect of Piper betle leaf extract against cadmium-induced oxidative stress and hepatic dysfunc-tion in rats. Saudi J Biological Sci. 2012; 19: 229-239.
- El-Demerdash FM, Yousef MI, Radwan FME.Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs.Food Chem Toxicol. 2009; 47: 249–254.
- Miura N. Individual susceptibility to cadmium toxicity and metallothionein gene polymorphisms with references to current status of occupational cad-mium exposure. Indust Health. 2009;47: 487–494.
- Masuda T, Maekawa T, Hidaka K, et al. Chemical studies on antioxidant mechanisms of curcumin: analysisof oxidative coupling products from curcu-min and linoleate. J Agric Food Chem. 2001; 49: 2539–2547.
- Daniel S, Limson JL, Dairam A, et al. Throughmetal binding, curcumin protects against lead-and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem.2004; 98 (2): 266-275.
- Kaur G, Tirkey N, Bharrhan S, et al. Inhibition of oxidative stress and cy-tokine activity by curcumin in amelioration of endotoxin-induced experimen-tal hepatotoxicity in rodents. Clin Exp Immunol. 2006; 145: 313–321.
- Reys-Gordillo K, Segovia J, Shibayama M, et al. Curcumin protects against acute liver damage in the rat by inhibiting NF-kB, proinflammatory cytokines production and oxidative stress. Biochem Biophys Acta. 2007; 1770: 989–996.
- Shapiro H, Ashkenazi M, Weizman N, et al. Curcumin ameliorates acute thioacetamide-induced hepatotoxicity. J Gastroenterol Hepatol. 2006; 21: 358–366.
- Tanwar V, Sachdeva J, Golechha M, et al. Curcumin protect rat myocardium against isoproterenol-induced ischemic injury: attenuation ofventricular dysfunction through increased expression of Hsp27 along with strengthening antioxidant defense system. J Cardiovasc Pharmacol. 2010; 55:377–384.
- Shapiro H, Ashkenazi M, Weizman N, et al. Curcumin ameliorates acute thio-acetamide-induced hepatotoxicity. J Gastroenterol Hepatol. 2006; 21:358–366.
- Sankar P, Telang AG, Manimaran A. Protective effect of curcumin on cyper-methrin-induced oxidative stress in Wistar rats. Exp Toxicol Pathol. in press.
- Tarasub N, Tarasub C,Devakul Na Ayutthaya W. Protective role of curcu-min on cadmium-induced nephrotoxicity in rats. J Environ Chem Ecotoxicol. 2011; 3 (2): 17-24.
- Carr AC, Frei B. Does vitamin C act as pro-oxidant underphysiological conditions? The Federation of American Societies for Experimental Biology. FASEB J.1999; 13: 1007–1024.
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clinic Care.2002; 5: 66–74.
- Sahin K, Kucuk O, Sahin N, et al. Effects of vitamin C and vitamin E on lipid peroxidation status, serum hormone, metabolite, and mineral concentra-tions of Japanese quails reared under heat stress (34 °C). Int J Vitam Nutr Res.2002; 72: 91–100.
- Yousef MI, Salem MH, Kamel KI, at al. Influence of ascorbic acid sup-plementation on the haematological and clinical biochemistry parameters of male rabbits exposed to aflatoxin B1. J Environ Sci Health B.2003;38 (2): 193–209.
- Yousef MI. Aluminium-induced changes in hemato-biochemical param- eters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicol. 2004; 199 (1): 47–57.