Promising Approaches to Mammalian Cell Culture of Chinese Hamster Ovary (CHO)
Received: 26-Aug-2023, Manuscript No. Jbclinphar-23-111293; Editor assigned: 28-Aug-2023, Pre QC No. Jbclinphar-23-111293 (PQ); Reviewed: 11-Sep-2023 QC No. Jbclinphar-23-111293; Revised: 18-Sep-2023, Manuscript No. Jbclinphar-23-111293 (R); Published: 25-Sep-2023
Citation: Akram AB. Promising Approaches to Mammalian Cell Culture of Chinese Hamster Ovary (CHO). J Basic Clin Pharma.2023,14(4):294-301
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
In the past 30 years, the mammalian cell cultures especially derived from humans and rodents, were and still the major vehicles for biologics production. Small molecules biologics are types of medications which include medicated proteins and viral vaccines which can be used for cancer treatment, protection and treatment of genetic diseases and hereditary problems. Most products generated from animal cell cultures have huge biotechnological values; they depend on conventional techniques in manufacture using microorganisms which resulted from genetic engineering. The flourish of new techniques allowed improving and developing to be made in the proper culture medium, modification of cells and bioreactors’ designing as well as operation. It is expected that they will be used commercially to connect these products with market places.
Keywords
Mammalian cell culture, Culturing tissues, Regenerative medicine
Abbreviations
CHO: Chinese Hamster Ovary, MAb: Monoclonal Antibody; DMEM: Dulbecco’ Modified Eagle’s Media; PBS: Phosphate Buffer Saline; BHK: Baby Hamster Kidney; CK: Canine Kidney; HEK: Human Embryonic Kidney
Introduction
Tissue culturing of mammalian cells are used for vaccine production through viral infection, and medicated proteins are produced through genetic engineering. They are very important for patients who have protein insufficiency or lacking. For example; diabetic patients, goiter patients, Duchenne Muscular dystrophy, dwarfism and Gaucher’s disease which is a genetic disorder characterized by decreased levels of functional enzyme called β-glucocerebrosidase, this ailment can be cured by cerezyme intake, which is a recombinant enzyme comes from mammalian cell culture [1].
Another example of therapeutic proteins is antibodies and specific binding proteins which neutralize molecules cause the diseases inside the body as the drug Etanercept which binds to Tumor Necrosis Factor (TNF), hence protecting it from inflammatory reaction in patients with rheumatoid arthritis [2].
The human cells specifically are poised for the sake of enabling chances in cell based therapies as well as regenerative medicine due to their ability to divide. Stem cells nowadays are derived from various sources and guide these cells to become specific cell types which can be used for different applications.
Cell line derivation and their normal life span
In the first stages of their development, mammalian cells are exposed to excessive proliferation and differentiation during development into various tissues and organs, for an adult, most of cells are quiescent; although they are characterized by metabolic activity and physiological role performance, like the physiological roles of kidney like filtration, reabsorption and excretion or the physiological roles of liver like synthesis, biotransformation and metabolism, most of cells do not divide actively, they divide in response to stimuli for the aim of replenishment of aged and damaged cells. Except epithelial cells as skin and intestinal cells which divide regularly regardless of the presence of stimuli or not.
There are more than 200 different types, some of them are difficult to be isolated and grown in specific cultures. The most cells amenable to be cultured are fibroblasts and specific epithelial cells (Figure 1).
The first step in isolating cells is explanting tissue in a suitable chemical and physical environment which should be suitable for these cells to survive and proliferate.
This required environment should contain a complicated mixture of nutrients, including sugars, amino acids, minerals and growth factors like insulin. Cells derived from tissues are anchorage dependent except for specific cell types in blood which means they do not divide as free- floating individual cells so; after releasing cells from tissue environment, cells need a suitable surface for the sake of attaching otherwise they will not be able to divide and will not survive.
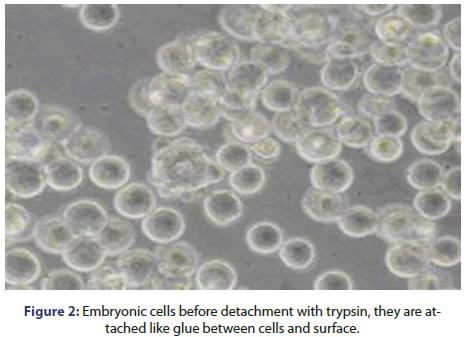
After cell attaching, cells divide and grow within the empty surface until they cover the entire surface and become a monolayer with a thickness of one cell. At this level, cells stop dividing and reach the contact inhibition state then trypsin enzyme is used for protein degradation of cells which are attached like glue between cells and surface, and hence the cells are released into the solution. As soon as detachment happens, the cells are transferred into a culture vessel with a bigger surface area to continue growth (Figure 2).
The cycle of attachment, cell division and cell detachment can be repeated multiple times, where each level includes cell growth. However, each type of cells has its own internal clock which counts its number of divisions. Cells stop dividing when they reach Hayflick limit [3,4]. Most cells divide from to times before stopping proliferation and get abnormal. Therefore, the number of doubling of these cells in culture is enough and applicable for vaccine production.
The contact inhibition of these cells is hallmark of cells isolated from normal tissues. Specific cells isolated from cancer are immortal and continue dividing ultimately without reaching contact inhibition cycle.
From more than 55 years ago, a successful attempt to isolate cells has been occurred which were survived senescence [5]. These cells divided although other cells died, by contrast of cancer cells, the surviving cells were normal morphologically and follow contact inhibition.
These types of cells bypassed Hayflick limit and divide ultimately called cell lines and they are immortal in their culture media, interestingly, they are unlike cell strains which are isolated from normal tissues. Cell strains and cell lines are different in an important point; all types of cell strain have normal chromosomes with two sets of chromosomes while the cell lines have not typical two sets of chromosomes, even if they are histologically and morphologically normal.
Most cells exhibits tumor when they are injected into immunecompromised mice. However, because they cultured ultimately, the cell lines are genetically engineered to produce unlimited quantities. So, all medicated proteins which are produced in mammalian cells using cell lines.
Stem cells
With the suitable chemical and physical environment, cells isolated from different tissues still have the same functions. For example; culture hepatocytes extracted from liver cells can retain its functional properties and continue albumin production and other functional proteins, at the same time still able to metabolize xenobiotics. But on the other hand these cells have a limited capacity to divide in culture media, but their limited growth removes their usage to be transplanted into patient for the aim of augmenting or replacement a diseased organ.
Most organs of human body have a small amount of stem cells which are capable to differentiate to be mature cells in the organ however, they are able to repair, maintenance or regeneration of tissues. Stem cells in adult tissues are restricted, as they can typically differentiate into cell of their own type. The most known type of stem cells is the hematopoietic cells found in bone marrow. Half a century ago, it was found that cells isolated from bone marrow can be transplanted into another patient to repopulate the constituent cells of the recipient.
Most organs of human body have a small amount of stem cells which are capable to differentiate to be mature cells in the organ however, they are able to repair, maintenance or regeneration of tissues. Stem cells in adult tissues are restricted, as they can typically differentiate into cell of their own type. The most known type of stem cells is the hematopoietic cells found in bone marrow. Half a century ago, it was found that cells isolated from bone marrow can be transplanted into another patient to repopulate the constituent cells of the recipient.
During the first stages of fetus development, when the fetus contains 70 to 100 cells, stem cells have the ability to differentiate in adult organs. This quality which is called pluripotency occurs before the fetus is implanted. These highly potent cells are transient; following a short period of expansion, they continue to be developed into different cell types in the embryo, and then lose their pluripotency character.
In the 1980s, mouse pluripotent cells were obtained from mouse embryo in its first stage and they were able to preserve its potency. These cells could be expanded in an undifferentiated state for more than 10 cell divisions and at the same time maintained their differentiation capacity to all cell lineages. Their chromosomes were very normal and diploid, like normal cell strains, but they represented a new type of cells. It took over 15 years until the embryonic cells of human were isolated and grown. This progress can offer great potential for regenerative medicine.
Biopharmaceuticals
The natural sources of biopharmaceuticals are rare and expensive as they are difficult to be kept up with demand, hard to isolate the product, lead to immune reactions and viral contamination so most of biopharmaceuticals are now recombinant so they become cheaper, safer due to avoidance of contamination and abundant supply as well as the expression occurs in isolated cells.
The advantages of mammalian cells
• Can express large protein with high molecular weight more than 50 kD.
• Glycosylation correction.
Note: Species differences between humans and rodents in the immunogenicity impact of product and its half life.
• Signal peptide removal.
• Proteins readily excreted.
• Have chaperonins to facilitate protein folding.
Uses of animal/human cells grown in culture
They are used as cell factory for biopharmaceuticals industry, Monoclonal Antibodies (MAbs), hormones as Follicle Stimulating Hormone (FSH), enzymes as α -glucosidase, vaccines as polio vaccine. It is used also test pharmaceutical drugs, observe disease mechanisms by designing potential treatments, observe the normal physiology and biochemistry of cells and to produce artificial tissues by combining different cell types as bone, cartilage and liver.
History of cell culture
• 1880-Roux Embryonic chicken cells in saline.
• 1900-Harrison-hanging drop technique (Figure 3).
Characteristics for in vitro cell growth established
• Anchorage: Cells require an anchor e.g. lymph clots on the cover slip.
• Nutrients: Which are provided by the lympgh.
• Slow growth rate: 20 hours doubling time compared to 20 minutes for bacteria which means that cell cultures are exposed to contamination.
• 1923-Carrel (surgeon)-It was the first cell culture which was developed with aseptic techniques.
• 1940/1950’s-Major Polio epidemics prompted development of polio vaccine (Figure 4).
This leaded to 2 important pioneering advances:
1. Primary monkey kidney cells
2. Human diploid lung fibroblast
These two progresses had tangible effects in bio-pharmaceutics production.
Culture requirements, culture vessels
The most important requirements are below:
• Adhesion: Borosilicate glass or sulphonated polystyrene.
• Temperature Equals 37°C: as the same temperature of human body.
• PH 7.4: the same acidity of blood, it can be maintained by using bicarbonates or CO2 buffer system (40 mm Hg partial pressure (5%)+24 mM HCO3 - in the medium).
• PH indicator: medium contains phenol red, the media looks pink/red at PH 7.2
N.B. phenol red is colored in acidic medium with yellow or orange which indicates cell growth and bacterial growth, and colored purple in basic medium which indicates no cell growth or not enough CO2 (Figure 5).
The standard medium used for mammalian cell culture is Dulbecco Modified Eagle’s Media (DMEM), it contains glucose, salts, amino acids, vitamins, antibiotics (Penicillin G 100 U/ml and streptomycin 50 mg/L) and amphotericin B 25 mg/L as an antifungal agent (Table 1).
| Components | Mole. weight | Conc. (mg/L) |
|---|---|---|
| Inorganic salts | ||
| Calcium chloride (CaCl2) (anhydrous) | 111 | 200 |
| Ferric nitrate (Fe (NO3)3.9H2O | 404 | 0.1 |
| Potassium chloride (KCl) | 75 | 400 |
| Magnesium sulfate (MgSO4) | 120 | 97.67 |
| Sodium chloride (NaCl) | 58 | 6400 |
| Sodium phosphate (NaH2PO4.H2O) | 138 | 125 |
| Other components | ||
| D-glucose | 180 | 4500 |
| Phenol red | 398 | 15 |
| Sodium pyruvate | 110 | 110 |
| Amino acids | ||
| L-arginine.HCl | 211 | 84 |
| L-cystine.2HCl | 313 | 63 |
| L-Glutamine | 146 | 584 |
| Glycine | 75 | 30 |
| L-Histidine.HCl.H2O | 210 | 42 |
| L-isoleucine | 131 | 105 |
| L-leucine | 131 | 105 |
| L-lysine.HCl | 183 | 146 |
| L-mehtionine | 149 | 30 |
| L-phenyl alanine | 165 | 66 |
| L-serine | 105 | 42 |
| L-threonine | 119 | 95 |
| L-tryptophan | 204 | 16 |
| L-tyrosine 2Na2H2O | 261 | 104 |
| L-valine | 117 | 94 |
| Vitamins | ||
| D-Ca pantothenate | 477 | 4 |
| Choline chloride | 140 | 4 |
| Folic acid | 441 | 4 |
| i-Inositol | 180 | 7.2 |
| Niacinamide | 122 | 4 |
| Pyridoxine HCl | 204 | 4 |
| Riboflavin | 376 | 0.4 |
| Thiamine HCl | 337 | 4 |
Note: Weight and concentrations (mg/L).
Table 1: Comparison Of General Information Of The Four Groups
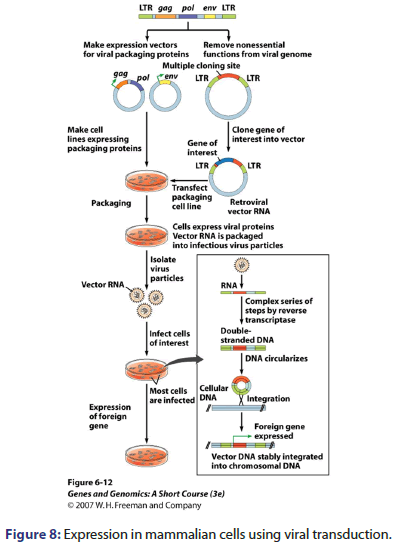
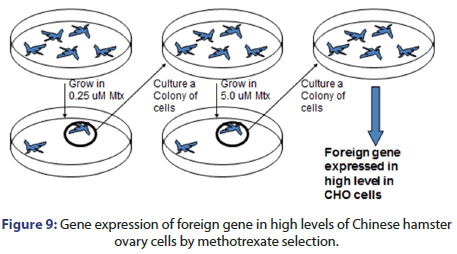
Expression of mammalian cells
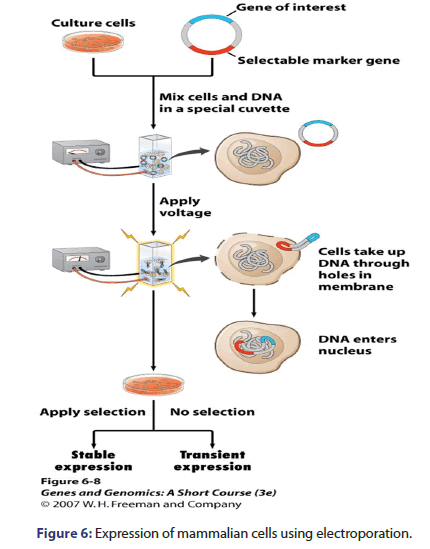
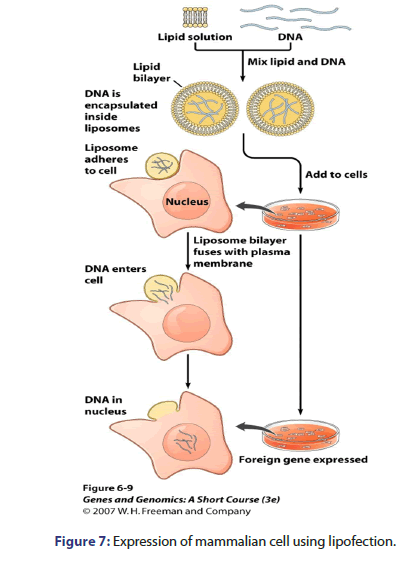
Mammalian cells are expressed by many techniques e.g. electroporation in which voltage is applied, lipofection which DNA is encapsulated inside liposomes, viral transduction which expression vector is made for viral packaging proteins and methotrexate selection as shown in Figures 6-9.
Most common cell lines
For mammalian tissue culture, although Chinese Hamster Ovary is the most known type of cultured cells, but there are many cell lines also used for tissue culture, some of them isolated from mice and many of them are isolated from different mammalian species which are detailed below in Table 2.
| Cell line | Origin organ isolated |
|---|---|
| BHK | Baby Hamster Kidney |
| CHO | Chinese Hamster Ovary |
| MDCK | Canine Kidney |
| Vero | Monkey Kidney |
| L929 | Mouse tumor |
| 3T3 | Mouse fibroblast |
| HeLa | Human cervical carcinoma |
| Namalwa | Human lymphoblastoma cell |
| MRC 5 | Human embryonic lung |
| HEK | Human Embryonic Kidney |
| PER.C6 | Human retinoblast |
Table 2: Most common cell lines illustrating organs which are isolated.
Parameters that affect expression
• Transcriptional efficiency: This affects from the chromosomal neighborhood of Tablethe transferred gene including promoters, enhancers, silencers, LCRs, SCSs and neighboring transcription in sense, structure of chromatin and CpG methylation islands. Promoter and enhancer of the transferred gene also affect expression and copy number of the transferred gene.
• RNA processing and transport: including capping, splicing, polyadenylation and transfer to cytoplasm.
• mRNA turnover: in which RNA sequences and peptide sequences of the translated RNA is affecting the stability of mRNA by shortening of the poly (A) tail and presence of cap.
• Translation efficiency: including secondary structures, cap, modification of translation factors, AUG context, 5’ UTR, distant sequences, binding of receptors of the mRNA, general translation status of the cell, poly (A) modification, presence of antisense RNA and translational masking.
Host cell modifications
The most promising approaches of host cell modifications are:
Enhancing viability of cells for example, anti-apoptotic proteins overexpression (Bcl-χL) c-myc; pro-apoptotic factors knockout as Bax and Bak [6].
• Cell cycle arresting induction.
• Reducing of inhibitory metabolic by-products as ammonia and lactate.
• Elevating density of the cell as overexpression of mTOR.
• Increase the capacity of protein secretion as overexpression of proteins involved in trafficking vesicle SNARE.
• Post-translational protein modification modulation [7].
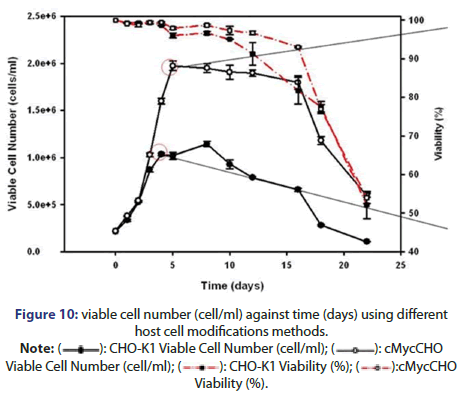
• Increasing proliferative viability and capacity: which can be done by: producing a cell line with high proliferation rate which can be grown to high densities, inducing transcriptional factor Myc which promotes cell proliferation and transformation by repression growth arrest genes expression or growth promoting genes activation and finally adherent CHO cell line transfection using c-myc gene which has been shown as a result of increasing cell number and proliferation rate (Figure 10) [8].
Cell line selection improvement and establishment of cell line with a single site of integration
This can be done by using homologous recombination to allow integration to a single location inside the genome of the cell line.
Materials and Methods
Passing cells
The general protocol for passing cells all solutions and equipment that come in contact with the cells must be sterile, the solutions and equipment are adapted from the Invitrogen guide to cell culture basics, always use proper sterile technique and work in a laminar flow hood.
The materials needed for cell culturing process are: pipette, microscope, conical tube, haemocytometer, conical flasks and cover slip.
The reagents which will be used are Dulbecco Modified Eagle Medium (DMEM), Phosphate Buffer Saline (PBS), trypsin, trypan blue and alcohol (Figure 11).
Procedure
• Remove and discard the spent cell culture media (DMEM+bovine serum+L-Glutamine) from the culture vessel using a pipette
• Wash cells using a balanced salt solution without calcium and magnesium. Gently add wash solution to the side of the vessel opposite the attached cell layer to avoid disturbing the cell layer, and rock the vessel back and forth several times.
o Note: The wash step removes any traces of serum, calcium and magnesium that would inhibit the action of the dissociation reagent
• Remove and discard the wash solution from the culture vessel
• Add the pre-warmed dissociation reagent such as trypsin to the side of the flask; use enough amount of reagent to cover the cell layer. Gently rock the container to get complete coverage of the cell layer
• Incubate the culture vessel at room temperature for approximately 2 minutes.
o Note: The actual incubation time varies with the cell line used
• Observe the cells under the microscope for detachment. If cells are less than 90% detached, increase the incubation time a few more minutes, checking for dissociation every 30 seconds. You may also tap the vessel gently to expedite cell detachment
• When 90% of cells or more have detached, tilt the vessel for minimal length of time to allow the cells to drain. Add the equivalent of 2 volumes (twice the volume used for the dissociation reagent) of pre-warmed complete growth medium. Disperse the medium by pipetting over the cell layer surface several times
• Transfer the cells to a 50 ml conical tube and centrifuge them for 5-10 minutes
o Note: The centrifuge speed and time vary based on the cell type
• Re-suspend the cell pellet in a minimal volume of pre-warmed complete growth medium and remove a sample for counting
• Determine the total number of cells and percent viability using a haemocytometer and trypan blue
o Note: If using culture flasks, loosen the caps before placing them to the incubator to allow proper gas-exchange unless you are using vented flasks with gas-permeable caps
Counting cells in a haemocytometer
• Clean the chamber and cover slip with alcohol. Dry and fix the coverslip in position
• Harvest the cells. Add 10 μl of the cells to the haemocytometer. Do not overfill
• Place the chamber in the inverted microscope under a 10X objective. Use phase contrast to distinguish the cells
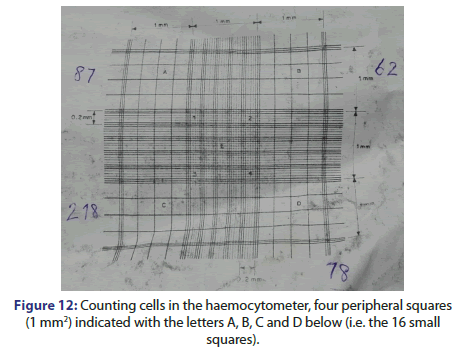
• Count the cells in the four peripheral squares (1 mm2) indicated with the letters A, B, C and D below (i.e. the 16 small squares)
• Divide this number by 4 and multiply by 104 to estimate the number of cells per ml (Figure 12).
Viable cell counts using trypan blue
Trypan blue is a vital dye. The reactivity of trypan blue is based on the fact that the chromophore is negatively charged and does not interact with the cell unless the membrane is damaged. Therefore, all the cells which exclude the dye are viable.
Procedure
• Place 0.5 ml of a suitable cell suspension (dilute cells in complete medium without serum to an approximate concentration of 1 × 105 to 2 × 105 cells per ml) in a screw cap test tube.
• Add 0.1 ml of 0.4% trypan blue stain and mix thoroughly.
• Allow to stand 5 minutes at room temperature.
• Fill a haemocytometer as for cell counting.
• Fill a haemocytometer as for cell counting.
Calculations for use with cultured cells
The observations at each passage of the cells will be mentioned in the results. The minimum information that should be recorded is:
• How confluent the culture is
• The cell titer per ml
• Total number of cells in the flask
This information allows determining how many cells to plate to give the required cell density after a specific time in culture.
The total number of cells in a flask is the cell titer per ml × the total volume of cells from which the aliquot was taken, (which is equal to confluent of the cells before they were passaged). From this the number of cells that equals a particular confluency can be obtained.
If the number of cells seeded is known as well as number of cells present upon passaging (e.g. after 48 hours) you can calculate how many cells needed to seed to give a required confluency at a particular time (e.g. 120 hours culture)
Results
Passing cells
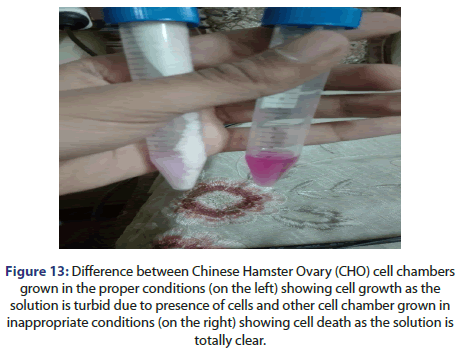
As showing in Figure 13 below it is found that the colour of dye in the chamber has been changed, the clear solution indicates no growth of cells after bringing it back from the incubator, when the cells are put in the proper temperature, it starts to grow but in the cases of storage in an inappropriate temperature it can lead to cell death, these cell chambers in the Figure below are obtained after 48 hours incubation (Figure 13).
Figure 13: Difference between Chinese Hamster Ovary (CHO) cell chambers
grown in the proper conditions (on the left) showing cell growth as the
solution is turbid due to presence of cells and other cell chamber grown in
inappropriate conditions (on the right) showing cell death as the solution is
totally clear.
Counting cells in a haemocytometer
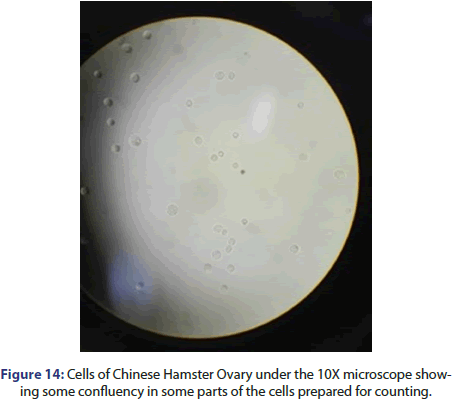
If you put the cell chamber containing clear solution under microscope you will not find any grown cells. As mentioned before, the cells are counted using a haemocytometer dye after not overfilling.
The chamber containing cells are put under an inverted microscope under a 10X objective and cells are counted in the four peripheral squares for each 1 mm2 indicated with the letters A, B, C and D as shown in Figure 14 below.
To estimate the number of cells per ml it is needed to count the cells in each section or Peripheral Square.
The count of viable cells in square A is 87 cells.
So, the number of cells in section A per ml=(count of cell in square A/4)*104=87/4*104=21.75*104 cell sper ml
The count of viable cells in square B is 62 cells.
The number of cells in section B per ml=(count of cell in square B/4)*104=62/4*104=15.5*104 cells per ml
The count of viable cells in square C is 218 cells.
The number of cells in section C per ml=(count of cell in square C/4)*104=218/4*104=54.5*104 cells per ml
The count of viable cells in square D is 78 cells.
The number of cells in section D per ml=(count of cell in square D/4)*104=78/4*104=19.5*104 cells per ml
The total number of cells in fla sk=217500+155000+545000+195000=111.25*104 cells per ml.
Discussion
Mammalian cell-line expression
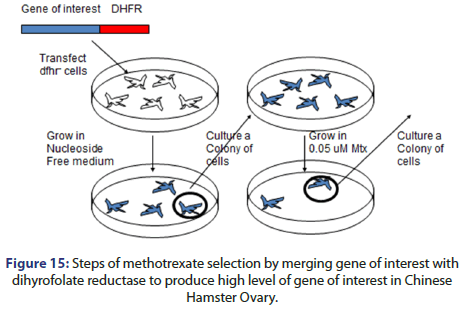
Cells are typically derived from Chinese Hamster Ovary (CHO) cell line. Vectors use SV-40 virus, CMV or vaccinia virus promoters and selectable marker gene e.g. Dihydrofolate Reductase (DHFR), glutamate synthetase and mammalian cells transformed by electroporation, with linear plasmid, and gene integrates, one or more times, into random locations within the different Chinese Hamster Ovary chromosomes [9].
Multiple rounds of growth and selection of the marker gene product are served to select for cells which have suitable expression of the gene of interest. The process can be done by methotrexate selection which done by:
• Hybridization the gene of interest with dyhydrofolate reductase then transfect the hybrid with dyhydrofolate reductase cells
• Grow cells in nucleoside free medium
• Then the cells colony is cultured to be grown in 0.05 μM methotrexate
• This leads to high gene of interest expression with high level in CHO (Figure 15).
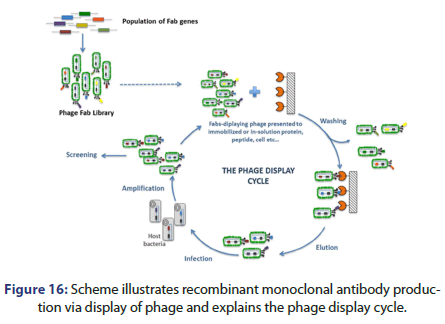
Recombinant monoclonal antibodies can be also produced by phage display; this can be done by creating Fab gene library to display the library on phage coats. Fab fragments are isolated against the antigen of interest then Fab fragment is cloned into a construct to form the whole antibody, the result is expression in human cell lines as PERC.6 or HEK293.
Figure 16 shows the phage display cycle. This can be done by the following:
• Fab genes population to form phage Fab library
• Fabs displaying phage presented to immobilized or in solution protein, peptide, cell etc.
• The cells are then washed and eluted then infected with the host bacteria to be amplified and screened and the cycle after that is repeated (Figure 16).
There are some suggestions to modify increased expression to target the gene of interest for example:
• Decreasing the intron coding region.
• Rare codons removal using the appropriate enzymes.
• Cryptic splice sites removal.
• Getting the optimum G/C content.
• Flank sequence with motifs which prevent chromatin condensation.
• Constitutive transport element inclusion in the 3’ un-translated region.
• Instability signals removal as AUUUA.
• Addition of N-terminal small ubiquitin-like modifier.
N.B. ubiquitin is a compound found in living cells that plays a vital role in the degradation of defective and superfluous proteins. It is a single chain polypeptide.
Mammalian cell culture at all has many important applications. Therapeutic proteins expressed in cell line can be expressed in wide range of mammalian cells including monoclonal antibodies, vaccines manufacture, growth factors, hormones and blood factors like biologics industry as cetuximab, adalimumab, infliximab, abatacept etc.
It can be also used for expression of factor IX, factor VIII, interferon gamma γ, interleukin 2, human Growth Hormone (GH), Tissue Plasminogen Activator (TPA), Follicle Stimulating Hormone (FSH) and erythropoietin.
Conclusion
Cell passaging can be done briefly by removing the liquid in the sample which is composed of (DMEM+Bovine serum+L-glutamine) then put 5 ml phosphate buffer saline and remove it then put again 5 ml phosphate buffer saline then remove it again then put 1 ml trypsin, finally put 4 ml of medium DMEM and incubate. The protocol should contain a complete list of the equipment, plastic ware and solutions. It should also contain a stepwise plan of the process that will be followed.
The exploration of promising approaches to mammalian cell culture of Chinese Hamster Ovary (CHO) cells represents a pivotal step forward in the field of biotechnology and pharmaceutical production. CHO cells are a cornerstone in the development of biotherapeutics and vaccines, making their cultivation efficiency a paramount concern. The research and innovations discussed in this context have illuminated several key strategies, such as advanced media formulations, genetic engineering, and bioprocess optimization, that have the potential to revolutionize CHO cell culture.
References
- Birch JR, Racher AJ. Antibody production. Adv Drug Deliv Rev. 2006;58(5-6):671-685.
[Crossref][Google Scholar] [PubMed]
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30(5):1158-1170.
- Matasci M, Hacker DL, Baldi L, et al. Recombinant therapeutic protein production in cultivated mammalian cells: current status and future prospects. Drug Discov Today Technol. 2008;5(2-3):e37-42.
[Crossref][Google Scholar] [PubMed]
- Stern, Pryme. Bioforum Europe. 2005:38-40.
- Le TS, McCann M, Azarin SM, et al. An introduction to mammalian cell culture. Chem Eng Prog. 2016 ;112(4):34-40.
- Xiao. Curr Opin Struct Biol. 2014:32–38.
- Lalonde ME, Durocher Y. Therapeutic glycoprotein production in mammalian cells. J Biotechnol. 2017;251:128.
[Crossref][Google Scholar] [PubMed]
- Kuystermans D, Dunn MJ, Al-Rubeai M. A proteomic study of cMyc improvement of CHO culture. BMC Biotechnol. 2010;10:1-3.
- Freshney RI. Culture of animal cells: a manual of basic technique. Alan R. Liss. Inc., New York. 1987;117.



 ): CHO-K1 Viable Cell Number (cell/ml); (
): CHO-K1 Viable Cell Number (cell/ml); ( ): cMycCHO Viable Cell Number (cell/ml); (
): cMycCHO Viable Cell Number (cell/ml); ( ): CHO-K1 Viability (%); (
): CHO-K1 Viability (%); ( ):cMycCHO Viability (%).
):cMycCHO Viability (%).