Preparation and In Vitro, In Vivo Evaluation of Clarithromycin Microcapsules
- *Corresponding Author:
- Liandong Hu
College of Pharmacy, Hebei University, Baoding 071002, China
E-mail: hbupharm@126.com
Date of Received :19-03-2010
Date of modified :29-05-2010
Date of Accepted :07-06-2010
Available Online :15-02-2011
Abstract
PURPOSE: To develop and validate a method to prepare clarithromycin (CLM) microcapsules to mask the bitter taste and provide effective treatment, and evaluate the quality of microcapsules in detail, especially the in vitro and in vivo pharmacoki-netics behavior. METHODS: CLM microcapsules were prepared using ethyl cellulose as matrix mate-rial by an emulsion solvent diffusion method. The physicochemical property, in vitro release study, sensory test and stability test were evaluated. Self-made CLM dry sus-pension or conventional tablets containing 250 mg of CLM were orally administered with 250 mL of water. The plasma concentration was determined and the pharma-cokinetic parameters were calculated by non-compartmental methods. RESULTS: Stable microcapsules could be prepared using ethyl cellulose as matrix material. The quality evaluation of prepared microcapsules was qualified, and the pharmacokinetic parameters of dry suspensions and conventional tablets were as following. Cmax were 1.32±0.62 and 1.40±0.58 μg•ml-1; Tmax were 3.51±0.54 and 2.01±0.42 h; AUC were 7.65±2.54 and 7.12±2.10 μg•h•ml-1. CONCLUSION: The preparation method is easy and applicable. The self-made CLM dry suspension containing microcapsules sufficiently alleviate the bitterness of commercial CLM dry suspension, but not decrease the bioavailability and have better effect for delaying drug release in healthy volunteers.
KeyWords
Clarithromycin; Microcapsules; Emulsion solvent diffusion method; Pharmacokinetics
Abbreviations
Clarithromycin (CLM) Ethyl cellulose (EC) Poloxamer 188 (F68) Potassium dihydrogen phosphate (KDP) Quality control (QC) Sodium carboxymethyl cellulose (CMC-Na)
Introduction
As every pharmacist knows, many pharmaceutical drugs have an unpleasant taste, often very bitter. The major consequence of the bitter taste is to restrict greatly the further development of oral preparations and clinical applications of these drugs. Along with the continuing improvement in the social standard of living, it is no longer acceptable for useful medicines to taste bitter. People wish to take effective drugs that have a nice taste and can be administered easily. Accordingly, it is important to mask the unpalatable taste of a drug in order to improve the product quality [1]. In order to achieve more pleasant dosage forms, various masking techniques have been described and microspheres techniques was one of the most popular method.
Microspheres are one of the multiparticulate delivery systems and are prepared to obtain prolonged or controlled drug delivery, to improve bioavailability or stability and to target drug to specific sites [2]. Microspheres can also offer advantages like limiting fluctuation within therapeutic range, reducing side effects, decreasing dosing frequency and improving patient compliance [3]. Various technologies have been proposed for fabricating microspheres, which include solvent evaporation [4,5], phase-separation [6], and spray-drying [7,8].
Clarithromycin (6-0-methyl-erythromycin A) is a 14-membered macrolide antimicrobial agent widely used for treatment of infections such as respiratory infection, skin soft tissue infection, choamydiae infection and helicobacter pylori infection and so on [9,10]. It is also clinically active against bacteria responsible for exacerbations of chronic bronchitis and the atypical pathogens that cause respiratory tract infections [11-13]. CLM was stable in the gastric acid and well absorbed, but it has a very bitter taste, this make it inconvenient when take orally.
In the production, it is hard to mask the bitter taste of CLM by simply adding correctives to the dry suspension and granules. Although it is able to mask the bitter by fluidized bed coating technique, the equipments have to consume considerable money and the operation is complicated. So it is necessary to search for a new way to prepare CLM dosage forms for masking the bitter. In this paper, The CLM microcapsules were prepared wiThethyl cellulose (EC) as matrix material by an emulsion solvent diffusion method. The quality evaluation of prepared microcapsules was qualified, and mixed with other adjuvant to prepare CLM dry suspension, and its pharmacokinetics in vivo was investigated with CLM conventional tablets as reference.
Materials,Instrumentation and Methods
Materials
Clarithromycin crude drug was purchased from Vitalpharms Company Ltd. (Zhuhai, China). CLM conventional tablet was kindly purchased from Shanghai Abbott Laboratories Ltd. (Shanghai, China). Commercial CLM granules were purchased from Yongan pharmaceutical Ltd. (Yunnan, China). Reference standard of CLM was provided by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). CLM microcapsules were self-made. EC (viscosity 20 cP) was purchased from Colorcon Ltd. (Shanghai, China). Ethyl acetate was obtained from Tianjin Xintong fine chemicals Ltd. (Tianjin, China). Poloxamer 188 and sodium carboxymethyl cellulose (CMC-Na, viscosity 200~500 cP, MW 600~800) were purchased from Xietai chemical agent Ltd. All other reagents and chemicals were of analytical grade and were obtained from the manufacturers.
Instrumentation
DF-101S constant temperature heating magnetic stirrer (Yuhua Instrument CO., LTD., Gongyi, China); FA-1104N electronic analytical balance (Shanghai minqiao precise scientific instrument limited company, Shanghai, China), Electron microscope (Chongqing optical instrument factory, Chongqing, China); AccuSizer780/DPS dry power particle size analysator (PSS LTD, American); D- 800L intelligence drug dissolution tester (Tianjin university radio company, Tianjin, China); P3000 analysis mode HPLC (including P3000 analysis mode pump, UV3000 ultraviolet detector, Beijing Chuangxin tongheng technology limited company, Beijing, China), JHH-150SD drug stability test apparatus (Yongsheng laboratory apparatus company, Chongqing, China).
The preparation of CLM microcapsules
The microcapsules were prepared by emulsion solvent diffusion method [14]. Briefly, the preparation involved five steps: (a) Preparation of oil phase: EC (1.0 g) and CLM (1.0 g) were dissolved in 20 ml of acetic ether. (b) Preparation of the aqueous phase: Poloxamer 188 (F68, 1 g) was dissolved in 200 ml double distilled water (saturated by acetic ether) to provide the aqueous phase of the emulsion. (c) Homogenization: the oil phase was quickly added to the aqueous phase with string at 230 r•min-1 for 5 min, then add 300ml water, keep the temperature at 10°, and string for 30 min. (d) Filter and wash the obtained solid with the double distilled water for 3 times. (f) The microcapsules were dried at 40° for about 6 h.
Particle size and morphology evaluation
Optical microscope was used to evaluate both the morphology and surface characteristics of the microcapsules. Particle size of the CLM microcapsules was determined by an AccuSizer780/DPS dry power particle size analysator before dispersing the microcapsules in deionized water.
Drug loading rate and entrapment rate
Validation of the optimized HPLC method was carried out with respect to the following parameters.
Chromatographic conditions
The HPLC separation was performed using a KromasilC18 (250 mm×4.6 mm, 5 μm). The mobile phase, consisting of potassium dihydrogen phosphate (KDP, 0.067 mol/l) –acetonitrile (6:4), was delivered at a flow-rate of 1.0 ml/min; Detection was set at 210 nm and the column temperature was maintained at 45°C. Injections were carried out using a 20 μl loop.
Linearity and range
Standard solution of CLM was prepared to a concentration of 3 mg/ml in a volumetric flask of 50 ml. Then dilute to 0.060 mg/ml, 0.12 mg/ml, 0.24 mg/ ml, 0.36 mg/ml, 0.48 mg/ml, 0.60 mg/ml and 0.72 mg/ml, respectively. Inject the sample of 20 μl, memorize the chromatogram. The different peak area of different concentration was drawn with the regression equation.
Precision
Inject the control sample in 5 times repeatly, and memorize the peak areas, and then calculate the RSD.
Reproducibility
Determine the same sample 5 times according to the method of 2.5.2 of this literature, and then calculate the RSD.
Sample analysis
Comminute CLM microcapsules and weight 40 mg (containing CLM 17.5 mg) of fine powders accurately, dissolve to a volumetric flask of 50 ml with the mobile phase using the ultrasound, then dilute to the scale, shake and filter. Take 20 μl of filter liquor accurately, inject and calculate the contents. Calculate the entrapment rate and drug loading rate with following formula [15]:

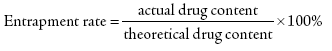
In vitro release study
CLM microcapsules (1120 mg equivalent to drug 500 mg) and crude drug (500 mg) were incubated in 900 ml of acetate buffer (pH 5.0) at 37±1°C under magnetic stirring at 100 r•min-1. Then it was determined according to China Pharmacopoeia 2005. At fixed time intervals an aliquot of suspension (5 ml) was withdrawn and filtered. Then the same volume of release medium is added. Take the filter liquor and determine according to the part of 2.5.2, then calculate accumulative release rate. Then the regression equations of release curves of CLM microcapsules are made.
Stability test
A short-term stability study was carried out at 40 °C, RH75% for 3 months.
Sensory Test
Based on clinical usage, dosage and specification of preparation, we definitely determine the prescription of dry suspension 250 mg per pouch. Take 11.2 g CLM microcapsules (containing CLM 5.0 g), 66.5 g sucrose, 25.0 g CMC-Na, 0.50 g sodium citrate, 0.50 g aspartame and mix together sufficiently. Pack 20 pouches. The prescription of dry suspension is 250 mg per pouch.
A single randomly study was designed for the sensory test in the buccal cavity. 18 volunteers participated in the sensory test. The 18 volunteers were divided into 2 sets blindly. Each set was 9 volunteers. Set 1 was administered commercial CLM dry suspension, and set 2 was administered self-made CLM dry suspension. One pouch of dry suspension was dispersed in 20 ml of water for 10 min. Immediately after preparation, each volunteer held about 1 ml of the dispersion in the mouth for 30 s. After expectoration, bitterness was evaluated.
Pharmacokinetics
Method validation
A microbiological cup-plate method was used in this part and the micrococcus luteus [CMCC(B)28001] was used as appreciation strain. To evaluate linearity, plasma calibration curves were prepared and assayed in triplicate on three consecutive validation days. Accuracy and precision were also assessed by determining QC samples at three concentration levels on the three different validation days. The accuracy was expressed by RE and the precision by RSD. The extraction recoveries of CLM at three QC levels were determined.
Application to a clinical pharmacokinetic study
The bioavailability study was in accordance with GCP/GLP standards. The protocol was approved by an ethics committee on bioavailability studies. The study was conducted on 12 healthy volunteers. Each volunteer was fully informed both in writing and verbally about the aim of the study. The 12 volunteers were divided into 2 sets randomly. Each set was 6 volunteers. Set 1 was administered one pouch of Self-made CLM dry suspension containing 250 mg CLM, and set 2 was administered one CLM conventional tablet containing 250 mg CLM after an overnight fast. Blood samples of 4 ml were collected just before and at 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after drug administration. The samples were placed in vacutainers (containing sodium heparin). Following centrifugation, the plasma samples were immediately frozen and stored (-18°C to -20°C) until assayed.
The pharmacokinetic parameters were calculated by non-compartmental methods. The AUC0-t was calculated using the trapezoidal method. Cmax and Tmax were obtained directly from the individual plasma concentration time profiles, ke was obtained as the slope of the linear regression of the log-transformed concentration versus time data in the terminal portion of the curve and t1/2 was calculated as 0.693/ke
Results And Discussion
Particle size and morphology evaluation
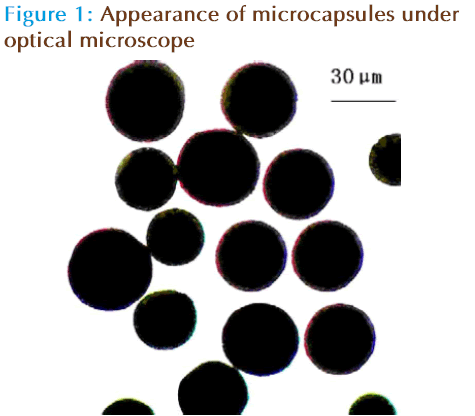
Under the optical microscope, the appearance of microcapsules was accord with requirement. Appearance of microcapsules under optical microscope was shown in Figure 1. The mean diameter of microcapsules was shown in Table 1. The micrographs show regular spherical shapes. It is apparent that the microspheres appear a smooth surface, welldistributed (mean diameter was 35.3μm) with no accretion among the microspheres.
| Batch number | Mean diameter /μm | Drug loading rate/% | Entrapment rate/% |
|---|---|---|---|
| 20070910 | 35.0±4.3 | 45.52 | 89.32 |
| 20070915 | 33.0±5.3 | 46.35 | 90.02 |
| 20070923 | 38.0±4.1 | 47.16 | 87.35 |
Table 1: Mean diameter of microcapsules, entrapment rate and drug loading rate in different batches (N=3)
Drug loading rate and entrapment rate
Linearity and range
The different peak area of different concentration was drawn with the regression equation: A = 11766+3003.2C, R=0.9999. With the result, the peak area and concentration was showed a fine linearity with a concentration range of 0.060-0.72 mg/ml.
Precision
Inject the control sample in 5 times repeatly, and memorize the peak areas, the RSD was 0.50%.
Reproducibility
Determine the same sample 5 times according to the method of 2.5.2 of this literature, the RSD was 0.31%.
Sample analysis
Three batches of microspheres were prepared based on the formulation, according to the method introduced above; the entrapment rate and drug loading rate were also shown in Table 1. The results indicated a high quality of the microspheres wiThentrapment rate and drug loading rate were (88.90±1.38)% and (46.34±0.82)% respectively.
In vitro release study
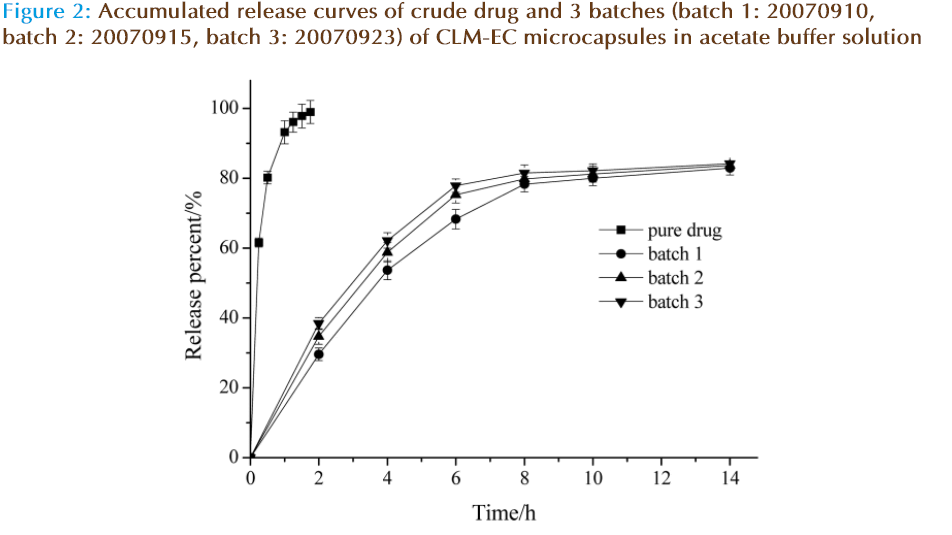
The dissolution rate of pure drug and the drug release rate from 3 batches of CLM microcapsules (batch number: 20070910, 20070915, 20070923) in acetate buffer solution were studied. The accumulated release curve was obtained in Figure 2. The result shows that compared to the pure drug, the self-made CLM microcapsules could sustain-release in vitro and the release rate was coincident among different batches. The rate of accumulated release is 75% on 6 h, meeting the design request.
Regression equations and correlation coefficients of different mathematical models of release curves of CLM microcapsules in vitro were showed in Table 2. According to the result, it was found that the dissolution rate of CLM microcapsules conformed to Higuchiequation and Peppas equation. The drug releasing mechanism of the preparation was chiefly characterized by drug diffusion including bulk erosion non-Fickian process.
| Model function | Regression equations | Coefficient correlation (R) |
|---|---|---|
| zero order release model | Y=3.3214t+40.143 | 0.9320 |
| first order release model | Ln(100-Y)=-0.0925t+4.1718 | 0.9577 |
| Higuchiequations | Y=18.233t 1/2+17.106 | 0.9825 |
| Peppas equations | LnY = 0.3995t + 3.4104 | 0.9896 |
Table 2: Regression equations of release curves of CLM microcapsules in vitro
Stability test
A short-term stability study was carried out at 40°C, RH 75% for 3 mouths and the results are shown in Table 3.
| Time(m) | Appearance under microscope | Drug loading(%) | Accumulative release (%) | ||
|---|---|---|---|---|---|
| 2hr | 4hr | 6hr | |||
| 0 | spherical morphology, smooth surface and no concretion | 46.5 | 37.9 | 60.1 | 78.4 |
| 1 | spherical morphology, smooth surface and no concretion | 46.8 | 37.2 | 61.5 | 76.9 |
| 2 | spherical morphology, smooth surface and no concretion | 46.3 | 38.6 | 62.8 | 77.6 |
| 3 | spherical morphology, smooth surface and no concretion | 46.1 | 38.1 | 60.8 | 77.8 |
Table 3: Stability of CLM microcapsules under accelerated test (N=3)
From Table 3, we can find that the appearance under microscope, drug loading rate and accumulative release rate were no significant changes. So it can be concluded that CLM microcapsules are stable for a period of 3 months under the accelerated test.
Sensory Test
The degree of bitterness was classified using 3 grades, corresponding to increasing bitterness, and a comparison of bitterness among samples was performed based on the total number of persons who selected “bitter” and “slightly bitter”. The ranking scheme used is shown in Table 4. The threshold of bitterness of Self-made CLM dry suspension was determined as the point at which half of the volunteers described the taste as bitter or slightly bitter. The results showed that the self-made CLM dry suspension sufficiently alleviate the bitterness of commercial CLM dry suspension (Table 4).This indicated that CLM was entrapment in the microcapsules; the bitter taste was masked efficiently in our research.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Set 1 | |||||||||
| No bitter | |||||||||
| Slightly bitter |
√ | √ | √ | √ | √ | √ | |||
| Bitter | √ | √ | √ | ||||||
| Set 2 | |||||||||
| No bitter | √ | √ | √ | √ | √ | √ | √ | ||
| Slightly bitter |
√ | √ | |||||||
| Bitter |
Table 4: Test of bitter taste
A research performed by Gao et al (1) was also for roxithromycin taste masking by microcapsule. Both as macrolide antibiotics, chemical structure and hydrophilicity (solubility) of CLM and roxithromycin are similar, so the authors focus on the difference of materials and methods in two papers.
In the research of Gao, Eudragit S100 was used as polymers material, mixture of ethanol, acetone and dichloromethane were used as solvents, distilled water was used as external phase, Ratio of drug and Eudragit S100 was 1:1. Microspheres of roxithromycin were prepared by the emulsion solvent diffusion method to mask the bitter taste.
In our study microcapsule were also prepared by the emulsion solvent diffusion method to mask the bitter taste, drug to polymers material (EC) was also 1:1, as we all know, the bitterness of macrolide antibiotics was clarithrmycin > Azithromycin > Roxithromycin. So a deeper study was done.
We investigate microcapsule using roxithromycin as a model drug using our materials and method, drug to EC was maintained at 3:1 and dry suspension was also made in the study. The result of sensory test showed that this roxithromycin formulation was not bitter. This indicated that the microcapsule prepared by our method, drug to polymers material EC can reach 3:1, the drug loading is higher than that by Gao’s (1:1).
In our study, the authors used ethyl acetate as solvent; the toxicity of ethyl acetate is smaller than that of acetone and dichloromethane reported by Gao’s.
Moreover, distilled water which presatured by ethyl acetate was used as external phase in our study, and distilled water was used by Gao. We also investigate microcapsule using distilled water as external phase during our study, but no microcapsule was obtained. This was because acetic ether is soluble in water, when oil phase was added, acetic ether in the oil phase can disperse to aqueous phase quickly, which made the EC floated in the solution to form a film, microcapsule can not obtained in this case. Distilled water presatured by ethyl acetate can avoid this by decreasing the disperse rate of acetic ether in aqueous phase. The morphology of microcapsule was integrity and yield was high.
Pharmacokinetics
Linearity and lower limit of quantification
CLM was showed linearity in the range of 0.01~5 μg•mL-1. Linear regression equation and correlation coefficient (R) were: lgC=0.1425D– 2.511(R=0.9986). The lower limit of quantitation was 0.01 μg•mL-1.
Precision and accuracy
Precision and accuracy were assessed by determining quality control (QC) samples at 0.02, 0.50 and 4.0 μg•mL-1 on three different validation days. The intra-run and inter-run precisions ranged from 2.7 % to 3.3 % and from 1.2 % to 1.5 % for each QC level, respectively. The accuracy was within 0.7%. The results, calculated using one-way ANOVA, indicated that the values were within the acceptable range and the method was accurate and precise.
Method selection
The maximum absorption wavelength of CLM was 210nm, so the specificity of HPLC-UV method for the determination of biological samples was not suitable. Some method including HPLC wiThelectrochemical detection [16] or amperometric detection [17], LC-MS/MS [18], have been published for the determination of in plasma samples. But these methods need a liquid-liquid extraction or solid phase extraction from the plasma, this made the operation so complicated and the instrument needed was expensive; so in this article, a microbiological method was used for the determination of CLM in human plasma. From the result of linearity, lower limit of quantification, precision and accuracy, we know that this method was accurate, convenient and sensitive for the estimation of pharmacokinetic parameter relative bioavailability of CLM dry suspension.
Application to a clinical pharmacokinetic study
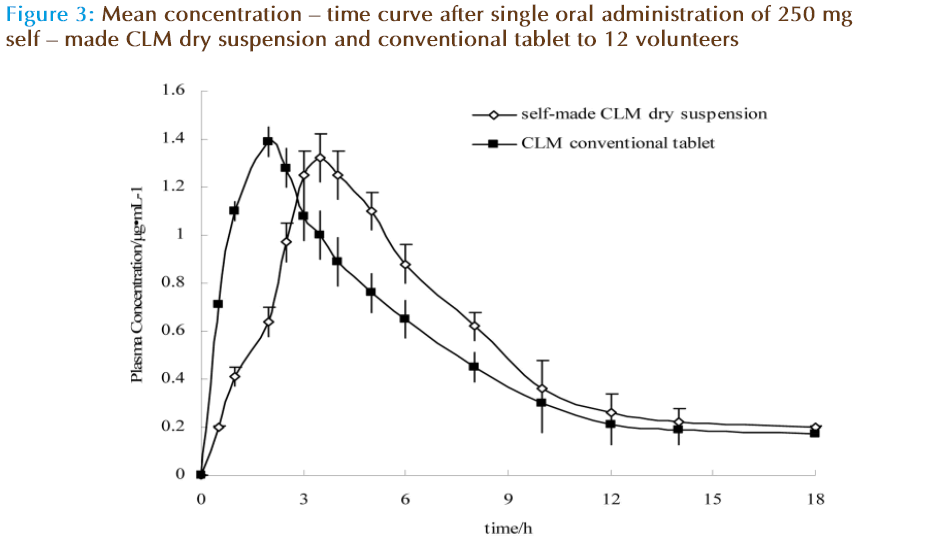
This method was adopted to determine the plasma concentration of Self-made CLM dry suspension and conventional tablet after a single oral administration of 250 mg CLM to 12 male volunteers. Mean plasma concentration-time profiles of Self-made CLM dry suspension and conventional tablet are depicted in Figure 3. Pharmacokinetic parameters are depicted in Table 5.
| parameters | Self-made CLM dry suspension | CLM conventional tablets | ||
|---|---|---|---|---|
| T1/2Ke/h | 3.54±1.32 | 4.21±1.86 | ||
| Tmax/h | 3.51±0.54 | 2.01±0.42 | ||
| C max/μg•ml-1 | 1.32±0.62 | 1.40±0.58 | ||
| AUC/μg•h•ml-1 | 7.65±2.54 | 7.12±2.10 | ||
Table 5: Pharmacokinetic parameters of CLM after an oral dose of 500 mg in 12 healthy volunteers
The pharmacokinetic parameters of dry suspensions and conventional tablets were as following. Cmax were 1.32±0.62 and 1.40±0.58 μg•ml-1; Tmax were 3.51±0.54 and 2.01±0.42 h; AUC were 7.65±2.54 and 7.12±2.10 μg•h•ml-1, T1/2 were 3.54±1.32 and 4.21±1.86, respectively. Cmax and AUC were not significantly different, but Tmax was significantly different. This indicated that the self-made CLM dry suspension containing microcapsules did not decrease the bioavailability and had better effect for delaying the release behavior significantly in healthy volunteers.
Conclusion
The rate of CLM release from these microcapsules into phosphate buffer appeared to be dependant upon a number of factors including differences in drug loading rate, drug solubility, microsphere surface area, surface morphology, hydrophobic nature and crystallinity of the polymer. The rate of polymer solidification appeared to be the main factor that determined the Drug loading rate.
According to the Optical microscope analysis, the prepared CLM microcapsules show a spherical morphology, a smooth surface, and no concretion. The dry suspensions of CLM have better effect for avoiding bitter taste and delaying drug release.
The established HPLC method is simple, rapid, suitable, sensitive, cost less and non-interference for the determination of plasma concentration of CLM and the study of pharmacokinetics.
References
- Gao Y, Cui FD, Guan Y, et al. Preparation of roxithromycin-polymeric microspheres by the emulsion solvent diffusion method for taste masking. Int. J. Pharm. 2006; 318: 62-69.
- Davis SS, Illum, L. Polymeric microspheres as drug carriers. Biomaterials. 1988; 9: 111–115.
- Ritschel WA. Biopharmaceutic and pharmacokinetic aspects in the design of controlled release peroral drug delivery systems. Drug Dev. Ind. Pharm. 1989; 15: 1073–1103.
- Bodmeier R, McGinity JW. The preparation and evaluation of drug containing poly (DL-lactide) microspheres formed by the solvent evaporation. Pharm. Res. 1987; 4: 465–471.
- Juni K, Ogata J, Nakano M, et al. Preparation and evaluation in vitro and in vivo of poly(lactic acid) microspheres containing doxorubicin. Chem. Pharm. Bull. 1985; 33: 313–318.
- Ruiz JM, Tissier B, and Benoit JP. Microencapsulation of peptide: a study of the phase separation of poly (D,L-lactic acid-co-glycolic acid) copolymers 50/50 by silicone oil. Int. J. Pharm. 1989; 49: 69–77.
- Bodmeier R, Chen H. Preparation of biodegradable poly lactide microparticles using a spray-drying technique. J. Pharm. Pharmacol. 1988; 40: 754–757.
- Wise DL, McCormick GJ, Willet GP, et al. Sustained release of an antimalarial drug using a co-polymer of glycolic / lactic acid. Life Sci. 1976; 19: 867–874.
- Langtry HD, Brogden RN. Clarithromycin: A review of its effcacy in the treatment of respiratory tract infections in immuo-competent patients. Drugs. 1997; 53(6): 973-1004.
- Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharma. 1999; 37(5): 385-398.
- Adam D. Clarithromycin 250 mg b.i.d. for 5 or 10 days in the treatment of adult patients with purulent bronchitis. Infection. 1993; 21: 265-271.
- Vincken W, Yemault JC. Efficacy and tolerability of clarithromycin versus azithromycin in the short-course treatment of acute bronchitis. Drug Invest. 1993; 6: 170-l 75.
- Wettengel R, Vetter N, and Waardenburg FA. Clarithromycin versus cefaclor for the treatment of mild-to-moderate acute bacterial bronchitis. J Antimicrob Chemother. 1993; 31: 963-972.
- Perumal D. Microencapsulation of ibuprofen and Eudragit RS 100 by the emulsion solvent diffusion technique. Int. J. Pharm. 2001; 218: 1-11.
- Qin LH, Tang X. Preparation and evaluation of clarithromycin emulsion for injection. Acta Pharmaceutica Sinica. 2006; 41(10): 945 – 949.
- Hedenmo M, Eriksson BM. Liquid chromatographic determination of the macrolide antibiotics roxithromycin and clarithromycin in plasma by automated solid phase extraction and electrochemical detection. J Chromatogr A. 1995; 692: 161-166.
- Taninaka C, Ohtani H, Hanada E, et al. Determination of erythromycin, clarithromycin , roxithromycin , and azithromycin in plasma by high performance liquid chromatography with amperometric detection. J Chromatogr B. 2000; 738(2): 405-411.
- Zhang XR, Chen XY, Li XY et al. Determination of Clarithromycin in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry: Validation and Application in Clinical Pharmacokinetic Study. Journal of Chinese Pharmaceutical Sciences. 2004; 13(3): 166-170.