Phytochemicals of Aristolochia tagala and Curcuma caesia exert anticancer effect by tumor necrosis factor-α-mediated decrease in nuclear factor kappaB binding activity
- *Corresponding Author:
- Dr. Lakhan Kma
Department of Biochemistry, Cancer and Radiation Countermeasures Unit, North-Eastern Hill University, Shillong - 793 022, Meghalaya, India.
E-mail: lakhonkma@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Rationale: The active compounds or metabolites of herbal plants exert a definite physiological action on the human body and thus are widely used in human therapy for various diseases including cancer. Previous studies by our group have reported the anticarcinogenic properties of the two herbal plants extracts (HPE) of Aristolochia tagala (AT) Cham. and Curcuma caesia (CC) Roxb. in diethylnitrosamine‑induced mouse liver cancer in vivo. The anticarcinogenic properties of these extracts may be due to the active compounds present in them. Objectives: Our objective was to analyze the phytochemical constituents present in AT and CC, to assay their antioxidant properties and to determine their role in a possible intervention on tumor progression. Materials and Methods: Qualitative and quantitative analysis of constituent with anticancer properties present in the crude methanol extract of the two plants CC and AT was carried out following standard methods. Separation of the phytochemical compounds was done by open column chromatography. The extracts were eluted out with gradients of chloroform‑methanol solvents. Ultraviolet‑visible spectra of individual fractions were recorded, and the fractions were combined based on their λmax. The free radical scavenging activity of crude extracts and fractions obtained was also determined; the radical scavenging activity was expressed as IC50. High‑performance thin layer chromatography (HPTLC) analysis of fractionated compounds was carried out to identify partially the phytochemical compounds. The anti‑inflammatory and anticancer activity of AT and CC extracts was studied in DEN induced BALB/c mice by analyzing the tumor necrosis factor‑α (TNF‑α) levels in serum and the nuclear factor kappaB (NF‑κB) binding activity in nuclear extracts of the liver. Results: It was observed that both AT and CC contained compounds such as phenolics, tannins, flavonoids, terpenoids, etc., and both extracts exhibited antioxidant capacity. HPTLC analysis revealed the presence of phenolic compounds in CC and indicated the presence of anthocynidin 3‑glycosides, 6‑hydroxylated flavonols, some flavones and chalcone glycosides in AT and also confirmed the presence of compounds such as terpenes, phenols, steroids, and other organic compounds in CC and presence of flavonoids in AT. In vivo studies carried out in BALB/c mice showed that exposure to DEN caused an increase in TNF‑α and NF‑κB binding activity. The HPE (CC or AT) was seen to revert this effect. Conclusions: The current paper documents the antioxidant, anti‑inflammatory, and anticancer activity of the two extracts probably through TNF‑α‑mediated decrease in NF‑κB binding activity. The active components of AT and CC may act as the potential anticancer agents in hepatocellular carcinoma and warrants further investigation.
Keywords
Antioxidant, Aristolochia tagala, Curcuma caesia, diethylnitrosamine, high performance thin layer chromatography, nuclear factor kappaB, tumor necrosis factor-α
Introduction
A prominent feature of physical, chemical, or biological carcinogens-induced carcinogenesis is through the induction of oxidative stress by increasing the production of reactive oxygen species (ROS). ROS plays an important role on DNA damage and induction of mutations, apoptosis as well as cell proliferation. Increased and compensatory cell proliferation takes place when there is extensive cell death and this process occurs through altered expression of cytokine growth factors such as tumor necrosis factor-α, interleukins (ILs), etc., and proto-oncogenes. [1,2] Compensatory cell proliferation can cause initiated cells to enter the cell cycle and transmit oncogenic mutations to their progeny leading to tumor promotion and progression. [3]
Tumor necrosis factor-α (TNF-α) have been shown to be a key mediator of inflammation and cancer. [4,5] TNF is a member of the TNF superfamily whose constituents are inducers of apoptosis, proliferation, nuclear factor kappaB (NF-κB), and c-Jun N-terminal kinase. NF-κB transcription factors are master coordinators of immune and inflammatory responses and play a major role in the protection from cell death through induction of expression of several anti-apoptotic proteins [6] and many other important mechanisms. [7,8]
As the underlying mechanism of carcinogenesis is perceived to be through oxidative damage caused by increase ROS within the system, it is expected that the increase of cancer incidence could be reversed by removing avoidable sources of ROS. Natural antioxidants are reported to have higher antioxidant activity that synthetic ones. Carotenoids, polyphenols, cinnamic acids, benzoic acids, folic acid, ascorbic acid, tocopherols, and tocotrienols are some of the antioxidants produced by the plant for their sustenance. [9] Previously, we have reported the chemopreventive potential of two herbal plants extracts (HPE) of Aristolochia tagala (AT) Cham. and Curcuma caesia (CC) Roxb. against diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) in mice by enhancing the antioxidant status and potentially protecting endogenous enzymatic and nonenzymatic antioxidant activity. [10] In this paper, we report the phytochemical constituents, free radical scavenging property, antiproliferative property of AT and CC, and the possible intervention on tumor progression.
Materials and Methods
Instrumentation and reagents
FeCl3, magnesium ribbon, concentrated HCl, concentrated sulfuric acid (H2SO4), chloroform (CHCl3), methanol, NaOH, Dragendorff’s reagent, Folin–Coicalteu’s reagent (10% [v/v] in water), NaHCO3, gallic acid, ascorbic acid, AlCl3, quercetine, iodine, anisaldehyde-sulfuric reagent, silica gel (100–200 mesh), were of analytical grade procured from various local sources. 1,1-diphenyl-2-picrylhydrazyl (DPPH), precoated silica gel 60 F 254 plates from Merck, Germany, glass column (10 mm × 300 mm), TNF-α Assay kit (Cayman Chemical, USA), NF-κB Transcription Factor Assay kit (Cayman Chemical, USA), Whatmann filter paper No. 1, Twin trough chamber, CAMAG high performance thin layer chromatography (HPTLC) System, Switzerland.
Qualitative phytochemical analysis of plant extracts
Most of the plant-derived natural products with documented anticancer/antitumor properties have been classified into major chemical groups such as alkaloids, flavonoids, terpenoids, phenols, and their derivatives. [11] We have, therefore, conducted the phytochemical test for these constituents. The qualitative analysis to test the presence of this constituent in the crude methanol extract of the two plants CC and AT were carried out following standard methods. [12-14]
Test for phenols and tannins
Crude extract was mixed with 2 ml of 2% solution of FeCl3. A blue-green or black coloration indicated the presence of phenols and tannins.
Test for flavonoids
Shinoda test
The crude extract was mixed with few fragments of magnesium ribbon and concentrated HCl was added dropwise. Pink scarlet color appeared after few minutes, indicated the presence of flavonoids.
Alkaline reagent test
The crude extract was mixed with 2 ml of 2% solution of NaOH. An intense yellow color was formed which turned colorless on the addition of few drops of diluted acid indicated the presence of flavonoids.
Test for steroid
The crude extract was mixed with 2 ml of CHCl3 and concentrated H2SO4 was added side-wise. A red color produced in the lower CHCl3 layer indicated the presence of steroids. Another test was performed by mixing the crude extract with 2 ml of CHCl3. Then, 2 ml of each of concentrated H2SO4 and acetic acid were poured into the mixture. The development of a greenish coloration indicated the presence of steroids.
Test for terpenoids
The crude extract was dissolved in 2 ml of CHCl3 and evaporated to dryness. To this, 2 ml of concentrated H2SO4 was added and heated for about 2 min. A grayish color indicated the presence of terpenoids.
Test for alkaloids
To a 2 ml of crude extract, 2 ml Dragendorff’s reagent (Solution A: 0.17 g bismuth nitrate in 10 ml 4:1 (water: Acetic acid), Solution B: 4 g potassium iodide in 10 ml water. Working solution was prepared by adding 5 ml Solution A, 5 ml Solution B, 20 ml Acetic acid and 70 ml water) was added. Prominent yellow precipitate indicated the presence of alkaloids.
Quantitative phytochemical analysis of plant extracts
The total phenolic content in the crude methanol extract of CC and AT was determined following standard methods described by Singleton and Rossi [15] and total flavonoid content in the crude methanol extract of CC and AT was determined following standard methods described by Quettier-Deleu et al. [16]
Total phenolic content
The total phenolic content of the extracts was estimated by preparing a reaction mixture containing 1 ml of methanol solution of extract CC or AT (1 mg/ml), 2.5 ml 10% Folin– Ciocalteu reagent, and 2.5 ml 2% Na2CO3. The mixture was mixed thoroughly and incubated in a thermostat at 45°C for 45 min and the absorbance of the sample read at 765 nm. A blank solution was prepared containing 1 ml methanol, 2.5 ml 10% Folin–Ciocalteu reagent, and 2.5 ml 2% Na2CO3. The samples were prepared in triplicates for each analysis, and the mean value of the absorbance was obtained. Gallic acid was used as a standard, and the same procedure was followed. The standard curve was constructed, and the concentration of phenolic in the plant extract was extrapolated from the standard curve. The results were expressed as Gallic acid equivalent (mg/g of extracted compound).
Total flavonoid content
The total flavonoid content of the extracts was estimated by preparing a reaction mixture containing 1 ml of a methanol solution of extract CC or AT (1 mg/ml) and 1 ml of 2% AlCl3. The samples were incubated for 1 h at r.t. and the absorbance was read at 415 nm. A blank solution was prepared containing 1 ml methanol and 1 ml 2% AlCl3. The samples were prepared in triplicates for each analysis, and the mean value of the absorbance was obtained. Quercetin was used as standard and the same procedure was followed. The standard curve was constructed and the concentration of flavonoids in the plant extract was extrapolated from the standard curve. The results were expressed as quercetin equivalent (mg/g of extracted compound).
Separation of phytochemical compounds of Curcuma caesia and Aristolochia tagala by open column chromatography
Separation of phytochemical compounds of CC and AT was carried out by open column chromatography according to the method describe by Stock and Rice [17] with minor modifications. The glass column was thoroughly washed with detergent, water and rinse with acetone to make grease free and dried. The packing material silica gel (10.5 g in 40 ml) was slurred with CHCl3 and poured into the column. A homogeneous packing of the silica gel was done by maintaining gentle agitation while there was a solvent flow through the column. 420 mg of crude extract was loaded into a column just above the filter paper soaked in the solvent kept to protect the column bed from any disturbance. When all the crude extract has been loaded on the top of the column, the vacant space above it was filled with solvent and the column was allowed to run. The supply of the solvent and combinations of the solvent system was replenished by gently loading 5 ml of solvent at a time with a pipette. The different solvents used were according to the procedure given by Harborne [18] with some modifications. The extracts were eluted out with gradients of CHCl3-methanol, 100 ml of 95:5 and 90:10 and 50 ml of 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, and 10:90 were used. Various fractions of 15 ml each were collected in clean test tube. Ultraviolet (UV)-visible spectra of individual fractions were recorded and the fractions were combined based on their λmax.
Free radical scavenging properties of crude methanolic extracts and fractions of Curcuma caesia and Aristolochia tagala
Free radical scavenging activity of extracts was determined by the method described by Williams et al. [19] with some modifications. 1 ml of methanol extract of plants (50, 100, 200, 400, 600, 800, 1000 μg/ml) was added to 2 ml of a solution of 0.004% (w/v) DPPH in methanol. The mixture was vortex vigorously and allowed to stand for 30 min at r.t. The absorbance was then read at 517 nm and converted into a percentage of antioxidant activity (AA%) using the following formula: AA% =100−([(Abssample − Absblank) ×100]/Abscontrol). 1 ml methanol and 2 ml of a solution of DPPH radical was used as a blank. Ascorbic acid was used as a standard and the same procedure was followed. The radical scavenging activity was expressed in terms of the amount of antioxidants necessary to decrease the initial DPPH absorbance by 50% (IC50). The IC50 value for each sample was determined graphically by plotting the percentage disappearance of DPPH as a function of the sample concentration.
Separation of fractionated compounds by high-performance thin layer chromatography
The fractionated compounds obtained after separation by column chromatography were again separated out by HPTLC according to the method described by Harborne [18] with minor modifications. HPTLC precoated plates (silica gel 60 F 254) were used. The plates were activated in an oven at 160°C for about 30 min before the samples were loaded. After complete cooling, the concentrated fractionated compounds were spotted onto the precoated plates using the CAMAG Linomat 5 automatic TLC sampler. The development chamber was prepared by pouring the solvent (CHCl3-MeOH; 9:1) into the glass chamber/tank to a depth of just > 0.5 cm. The chamber/tank was covered tightly and kept for 30 min until the chamber was saturated with the solvent. The plates were then allowed to develop in the chamber until the solvent run was about 5/6 of the plate. After the plates were developed, they were removed from the chamber and viewed using the CAMAG UV visualizer at 254 nm and 366 nm and then scanned using the CAMAG TLC scanner 4 and photographed. The plates were then derivitized using different derivitizing agents, such as Folin–Colcateau reagent, anisaldehyde-sulfuric reagent (5 ml of H2SO4 added to an ice cooled mixture of 85 ml of methanol and 10 ml of glacial acetic acid. To this solution, 0.5 ml of anisaldehyde solution was added with consistent shaking), AlCl3 and DPPH to determine the compounds present. The plates were then viewed, scanned at 700 nm and photographed.
Experimental animals
Albino BALB/c mice, aged 6–8 weeks from the inbred colony maintained in an animal house 20°C ± 2°C and 12 h light and 12 h dark conditions were used in all experiments. They were provided with a standard mouse pellets and drinking water ad libitum. The experimental protocols were approved by the Institutional Ethics Committee of North-Eastern Hill University, Shillong, India.
Experimental protocols
DEN was used to induce HCC in mice. Methanolic extracts of roots of AT and rhizomes of CC HPE were prepared. [10] DEN was administered by intravenous route [10] at weekly intervals from week 0 (start of the experiment) until the end of the experiment (16 or 28 weeks). The exposures to the HPE (either AT or CC) were initiated from week 10 until the end of the experiment (weeks 16 or 28) by thrice weekly intraperitoneal (i.p.) injections. [10]
Assay of tumor necrosis factor-α level in serum
Blood was collected by retro-orbital bleeding and centrifuged at 2000 ×g for 20 min at 4°C to separate the serum. The level of TNF-αin serum was determined following the instructions provided in the TNF-α Assay kit from Cayman Chemical, USA.
Assay of DNA binding activity of nuclear factor kappaB (p65) in nuclear extract
Nuclear extract from whole homogenate of the liver (20%, w/v) of experimental mice and age-matched control mice were prepared according to the instructions provided in the kit. The DNA binding activity of NF-κB was determined following the instructions provided in the NF-κB Transcription Factor Assay kit from Cayman Chemical, USA.
Data and statistical analysis
All data presented are the mean ± standard deviation of three independent experiments. Statistical analysis was performed by GraphPad Prism 6 software, (GraphPad Software, Inc., San Diego, California, USA) using one-way ANOVA followed by the Tukey’s multiple comparisons test. Significance was set at P < 0.05.
Results
Qualitative analysis
The phytochemical characteristics of the two plant extracts are summarized in Table 1. The results revealed the presence of medically active compounds in the two plants studied. AT showed the presence of phenolics, tannins, flavonoids and steroids and CC showed the presence of phenolics, tannins, flavonoids, terpenoids, and alkaloids in their crude methanolic extracts.
| Bioactive compounds | Methanol extract | |
|---|---|---|
| Aristolochia tagala | Curcuma caesia | |
| Phenolics/tannins | + | + |
| Flavonoids | + | + |
| Terpenoids | - | + |
| Alkaloids | - | + |
| Steroids | + | + |
Table 1: Qualitative analysis of plants showing presence of different bioactive compounds in crude methanolic extracts of Curcuma caesia and Aristolochia tagala
Quantitative analysis
Total phenolic content and total flavonoid content
The total phenolic content of the methanolic extracts obtained were 52.96 mg/g and 37.45 mg/g of the extract for AT and CC, respectively and the total flavonoid content of the methanolic extracts obtained were 51.6 mg/g and 10.4 mg/g of the extracts of AT and CC, respectively [Table 2]. AT, therefore, harbors a higher phenolic and flavonoid content than CC.
| Methanol extract | Gallic acid equivalent (mg/g extract) | Quercitin equivalent (mg/g extract) |
|---|---|---|
| Aristolochia tagala | 52.965±0.415 | 51.645±0.115 |
| Curcuma caesia | 37.45±0.01 | 10.435±0.055 |
Table 2: Total phenolic and flavonoid content of crude methanolic extract of Aristolochia tagala and Curcuma caesia
Separation of phytochemical compounds of Curcuma caesia and Aristolochia tagala by open column chromatography
Thirty-eight fractions of 15 ml each were collected from methanolic extracts of CC and AT after they were subjected to silica gel column chromatography. The fractions were pooled based on their respective absorption maxima (λmax nm) into 3–5 major fractions [Table 3].
| Fractions | λmax (nm) |
|---|---|
| (a) | |
| I | 323 |
| II | 345 |
| III | 308 |
| IV | 296 |
| V | 330 |
| (b) | |
| I | 323, 395 |
| II | 323, 258 |
| III | 323 |
Table 3: Major fractions of Curcuma caesia (a) and Aristolochia tagala (b) separated using silica gel open column chromatography and their respective absorption maxima (λmax nm)
The major fractions obtained from the separation of CC and AT and their respective absorption maxima (λmax nm) as shown in Table 3a and b. Five major fractions were obtained for CC and three major fractions were obtained for AT.
Free radical scavenging properties of crude methanolic extracts and major fractions of Curcuma caesia and Aristolochia tagala
To assess the free radical scavenging potential of the crude extract and fractions of CC and AT, the reactivity toward the stable free radical DPPH, was measured. The absorbance was converted into an AA%. The results are expressed as IC50 value. A lower IC50 value corresponds to a high antioxidant property and vice versa.
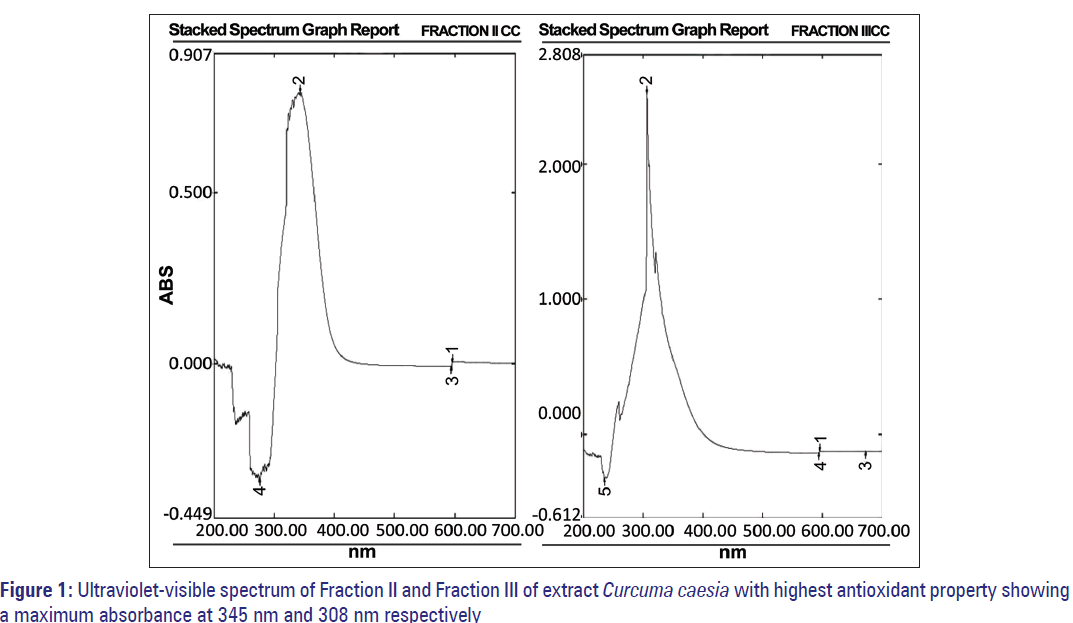
CC showed an IC50 value of 230 μg/ml. Its major fractions showed variable AA% at 200 μg/ml concentrations, the highest being Fraction II and III at 92.8% and 91.99% corresponding to an IC50 value of 108.70 and 107.75 μg/ml, respectively [Table 4a and b]. The absorption maxima (λmax nm) of Fraction II was 345 nm and Fraction III was 308 nm [Figure 1].
| (a) | |||
| Methanol extract | IC50 (µg/ml) | ||
| Curcuma caesia | 229.225±3.459 | ||
| (b) | |||
| Fractions | λmax (nm) | AA % inhibition at 200 µg | IC50 (µg/ml) |
| I | 323 | 64 | 156.25 |
| II | 345 | 92.8 | 107.75 |
| III | 308 | 91.99 | 108.70 |
| IV | 296 | 19.7 | 507.61 |
| V | 330 | 40.73 | 245.51 |
Table 4: Free radical scavenging property of crude methanolic extract (a) and Fractions I-V (b) of Curcuma caesia
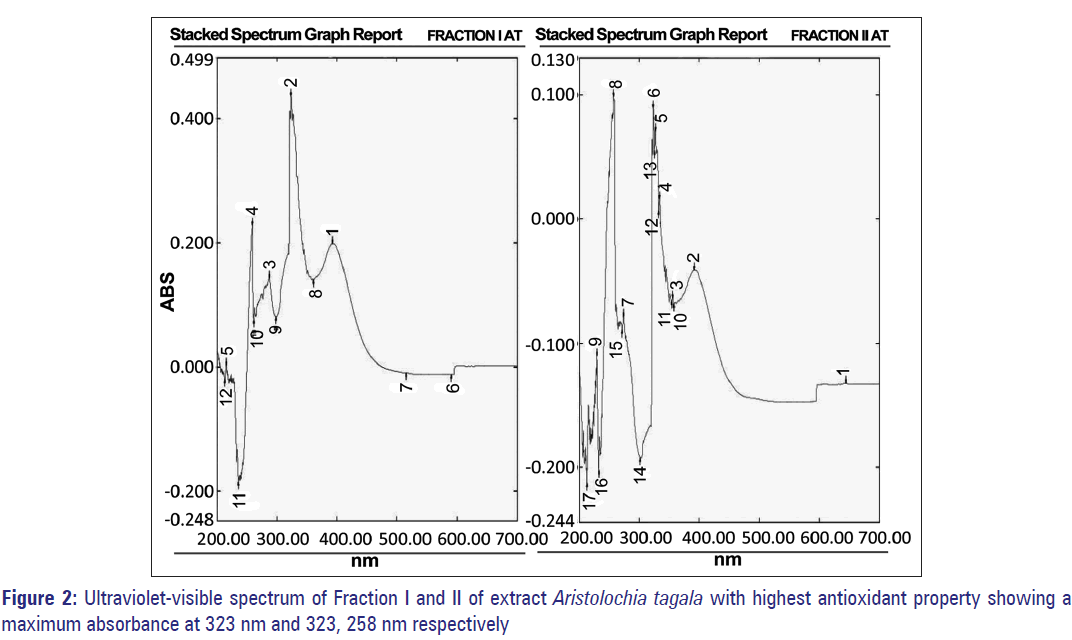
AT showed an IC50 value of 414.2 μg/ml. Its major fractions showed variable AA% at 400 μg/ml concentrations, the highest being Fraction I and II at 83% and 66% corresponding to an IC50 value of 240.03 and 302.1 μg/ml, respectively [Table 5a and b]. The absorption maxima (λmax nm) of Fraction I was 323 nm and Fraction III was 323 and 258 nm [Figure 2].
| (a) | |||
| Methanol extract | IC50 (µg/ml) | ||
| Aristolochia tagala | 414.2±1 | ||
| (b) | |||
| Fractions | λmax (nm) | AA % inhibition at 400 µg | IC50 (µg/ml) |
| I | 323, 395 | 83.2 | 240.03 |
| II | 323, 258 | 66.2 | 302.1 |
| III | 323 | 35.6 | 561.7 |
Table 5: Free radical scavenging property of crude methanolic extract (a) and Fractions I-III (b) of Aristolochia tagala
Separation and identification of compounds of Curcuma caesia and Aristolochia tagala by high performance thin layer chromatography
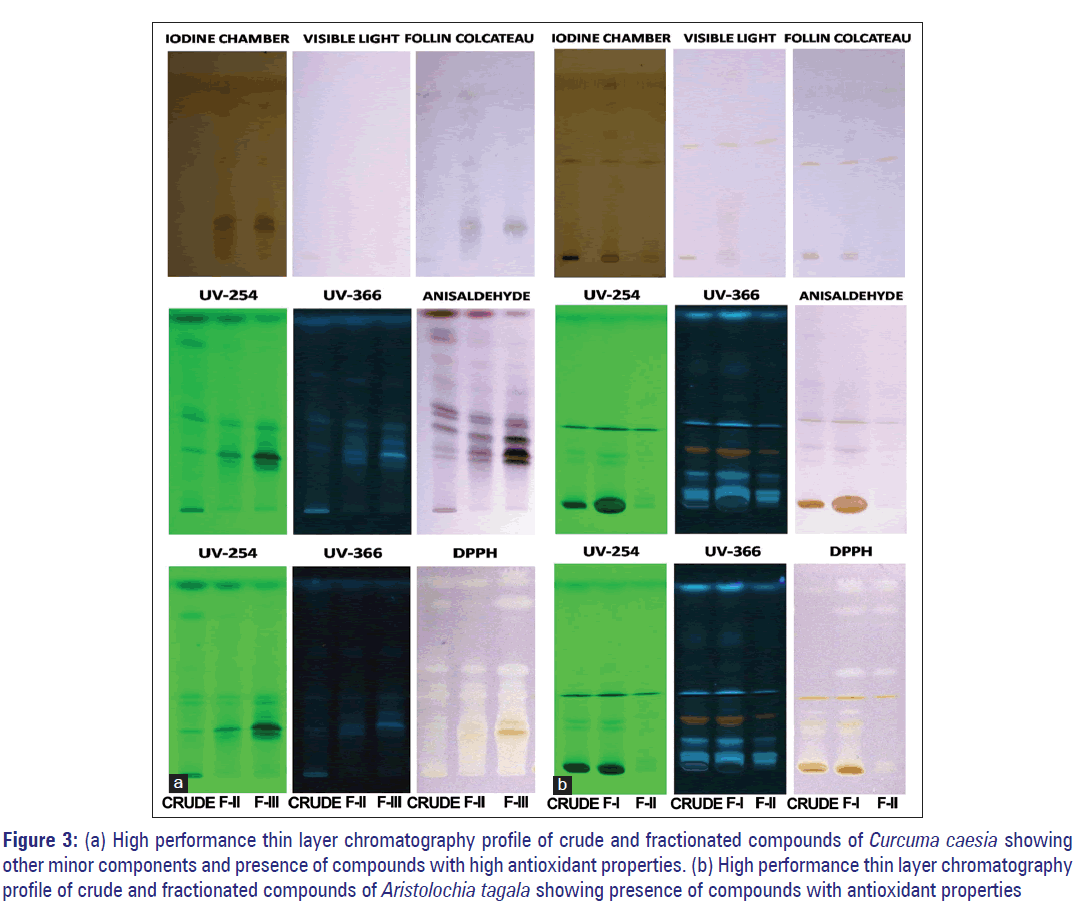
Separation of fractionated compounds of CC by HPTLC revealed the presence of two spots when placed in iodine chamber. When viewed in UV 254 dark brown spots were observed [Figure 3a]. After spraying with Folin reagent blue spots appeared, which indicates phenolic compounds [Figure 3a]. It was also observed that compounds of CC not all seen under UV light became visible with anisaldehyde spray. The HPTLC analysis revealed that the extract contains a large number of minor components. A number of spots as seen from the HPTLC profiles [Figure 3a] corresponding to different RF values as shown in Table 6a-c were detected by HPTLC analysis. This confirms the presence of compounds such as terpenes, phenols, steroids, and other organic compounds that showed positive results in qualitative analysis. The spots also showed free radical scavenging property when sprayed with DPPH radical. Faint yellow spots (zone of antioxidant activity) were seen on a light purple background [Figure 3a]. Based on their RF values, these spots have been equated with the spots that were observed when sprayed by different reagents as mentioned earlier [Figure 3a and Table 6]. From the spectral properties of phenolic compounds, dark-brown color observed in UV light, which appeared as blue when sprayed with Folin–Ciocalteau reagent indicates the presence of catechol or hydroquinone. [18] The profile also revealed evidently the presence of antioxidants constituents at RF values of 0.22, 0.27, 0.32, 0.43, 0.64, 0.82, and 0.94 cm [Figure 3a and Table 6c].
Figure 3: (a) High performance thin layer chromatography profile of crude and fractionated compounds of Curcuma caesia showing other minor components and presence of compounds with high antioxidant properties. (b) High performance thin layer chromatography profile of crude and fractionated compounds of Aristolochia tagala showing presence of compounds with antioxidant properties
| RF values (a) |
|||||||||
| IC 700 | FC 700 | ||||||||
| Crude | F-II | F-III | Crude | F-II | F-III | ||||
| 0.05 | 0.23 | 0.22 | 0.04 | 0.22 | 0.23 | ||||
| 0.25 | 0.27 | ||||||||
| 0.36 | 0.36 | 0.30 | 0.30 | ||||||
| 0.43 | 0.43 | ||||||||
| 0.55 | 0.54 | ||||||||
| 0.68 | 0.67 | 0.64 | 0.67 | ||||||
| 0.90 | 0.90 | 0.92 | 0.94 | 0.90 | |||||
| 0.98 | 0.98 | ||||||||
| (b) | |||||||||
| Crude | F-II | F-III | |||||||
| 254 | 366 | 700 | 254 | 366 | 700 | 254 | 366 | 700 | |
| 0.18 | 0.05 | 0.19 | 0.05 | 0.06 | |||||
| 0.24 | 0.25 | 0.25 | 0.26 | 0.27 | 0.23 | ||||
| 0.26 | 0.27 | 0.28 | 0.30 | ||||||
| 0.33 | 0.30 | 0.35 | 0.31 | 0.35 | 0.34 | 0.38 | 0.34 | 0.37 | |
| 0.45 | 0.45 | 0.40 | 0.38 | 0.42 | 0.37 | 0.41 | 0.44 | ||
| 0.49 | 0.46 | 0.47 | 0.49 | 0.50 | |||||
| 0.52 | 0.52 | 0.54 | 0.58 | 0.54 | |||||
| 0.67 | 0.68 | 0.63 | 0.68 | 0.65 | 0.66 | 0.65 | 0.64 | 0.63 | |
| 0.72 | 0.79 | 0.75 | 0.74 | 0.72 | |||||
| 0.84 | 0.87 | 0.85 | 0.85 | 0.87 | |||||
| 0.91 | 0.91 | ||||||||
| 0.96 | 0.95 | 0.95 | 0.98 | 0.98 | 0.94 | 0.98 | 0.98 | ||
| (c) | |||||||||
| Crude | F-II | F-III | |||||||
| 254 | 366 | 700 | 254 | 366 | 700 | 254 | 366 | 700 | |
| 0.19 | 0.20 | 0.10 | 0.07 | 0.19 | |||||
| 0.22 | 0.23 | 0.22 | 0.24 | 0.22 | |||||
| 0.26 | 0.28 | 0.25 | 0.27 | 0.26 | 0.27 | 0.26 | |||
| 0.30 | 0.30 | 0.29 | |||||||
| 0.35 | 0.35 | 0.32 | 0.32 | ||||||
| 0.38 | 0.39 | 0.40 | 0.40 | 0.40 | 0.37 | ||||
| 0.43 | 0.43 | 0.43 | 0.43 | 0.44 | 0.43 | ||||
| 0.47 | 0.47 | ||||||||
| 0.56 | 0.57 | 0.57 | |||||||
| 0.64 | 0.63 | 0.67 | 0.64 | 0.64 | 0.67 | 0.63 | 0.64 | 0.65 | |
| 0.80 | 0.81 | 0.80 | 0.80 | 0.83 | 0.82 | ||||
| 0.94 | 0.90 | 0.95 | 0.94 | 0.90 | 0.94 | 0.94 | 0.91 | 0.93 | |
| 0.99 | 0.98 | 0.99 | 0.99 | 0.93 | |||||
Table 6: RF values of corresponding spots observed
from the high performance thin layer chromatography
profile of fractionated compounds of Curcuma caesia
kept in (a) iodine chamber and sprayed with Folin–
Ciocalteau reagent, (b) sprayed with anisaldehyde, (c)
sprayed with 1,1-diphenyl-2-picrylhydrazyl sprayed
reagent
The compounds of AT, on the other hand, were clearly visible under UV light. The developed and dried plates observed under UV light of both long and short wavelength (254 nm and 366 nm) detected spot/band. The HPTLC analysis revealed that some of the compounds appeared as fluorescent spots while the others as dark spots under UV light [Figure 3b] corresponding to different RF values as shown in Table 7a-c. The profile further confirms the possible presence of flavonoids by revealing the fluorescent bands. The profile revealed light blue, bright blue, dull orange-red, fluorescent band and dark brown, band at RF 0.08, 0.16, 0.25, 0.70, 0.99, 0.27, 0.33, and 0.49 cm, respectively, under the TLC scanner at 366 nm [Figure 3b]. Based on the spectral properties of flavonoids, dull orange, red, or mauve under UV, which appears orange or faint red visually, indicates anthocyanidin 3-glycosides. A dark brown or black spot, which appears bright yellow visually, indicates the presence of 6-hydroxylated flavonols, flavones, or chalcone glycosides. [18] The profile also revealed more evidently that some of these compounds exhibited antioxidants properties at RF values of 0.25, 0.33, 0.43, 0.049, 0.64, 0.99 cm through the DPPH spray reagent [Figure 3b and Table 7c].
| RFvalues (a) | ||||||||||||
| IC (700) | 700 | FC (700) | ||||||||||
| Crude | F-I | F-II | Crude | F-I | F-II | Crude | F-I | F-II | ||||
| 0.03 | 0.04 | 0.04 | 0.03 | 0.02 | 0.02 | 0.02 | ||||||
| 0.13 | 0.30 | |||||||||||
| 0.42 | 0.41 | 0.40 | 0.46 | 0.45 | 0.49 | 0.40 | 0.41 | 0.43 | ||||
| 0.63 | 0.63 | |||||||||||
| 0.82 | 0.73 | |||||||||||
| 0.93 | ||||||||||||
| (b) | ||||||||||||
| Crude | F-I | F-II | ||||||||||
| 254 | 366 | 700 | 254 | 366 | 700 | 254 | 366 | 700 | ||||
| 0.03 | 0.03 | |||||||||||
| 0.05 | 0.06 | 0.07 | 0.05 | 0.06 | 0.06 | 0.06 | ||||||
| 0.10 | 0.09 | 0.10 | 0.12 | 0.11 | ||||||||
| 0.18 | 0.15 | 0.14 | 0.15 | |||||||||
| 0.21 | 0.25 | 0.24 | 0.24 | 0.20 | ||||||||
| 0.26 | 0.28 | 0.29 | 0.27 | 0.27 | 0.29 | |||||||
| 0.31 | 0.30 | 0.34 | 0.32 | 0.33 | 0.31 | 0.33 | ||||||
| 0.38 | 0.38 | 0.37 | 0.38 | 0.37 | 0.38 | |||||||
| 0.47 | 0.41 | 0.46 | 0.47 | 0.46 | 0.40 | |||||||
| 0.54 | 0.53 | 0.54 | 0.52 | 0.52 | 0.52 | |||||||
| 0.60 | 0.63 | 0.62 | ||||||||||
| 0.69 | 0.69 | |||||||||||
| 0.73 | 0.74 | 0.72 | 0.73 | |||||||||
| 0.87 | 0.90 | 0.88 | 0.83 | |||||||||
| 0.98 | 0.99 | 0.98 | 0.99 | 0.98 | ||||||||
| (c) | ||||||||||||
| Crude | F-I | F-II | ||||||||||
| 254 | 366 | 700 | 254 | 366 | 700 | 254 | 366 | 700 | ||||
| 0.06 | 0.06 | 0.07 | 0.07 | 0.05 | ||||||||
| 0.08 | 0.08 | 0.08 | 0.08 | |||||||||
| 0.18 | 0.16 | 0.11 | 0.18 | 0.16 | 0.17 | |||||||
| 0.20 | 0.23 | 0.24 | ||||||||||
| 0.25 | 0.25 | 0.25 | ||||||||||
| 0.27 | 0.28 | 0.26 | 0.27 | 0.28 | ||||||||
| 0.31 | 0.31 | 0.33 | 0.33 | 0.30 | ||||||||
| 0.35 | 0.34 | 0.35 | 0.34 | |||||||||
| 0.38 | 0.39 | 0.39 | 0.39 | 0.40 | ||||||||
| 0.42 | 0.42 | 0.42 | 0.43 | |||||||||
| 0.47 | 0.48 | 0.49 | 0.47 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 | ||||
| 0.56 | 0.55 | 0.61 | 0.59 | |||||||||
| 0.63 | 0.64 | 0.65 | ||||||||||
| 0.70 | 0.70 | 0.64 | 0.66 | |||||||||
| 0.77 | 0.79 | 0.74 | ||||||||||
| 0.82 | ||||||||||||
| 0.90 | ||||||||||||
| 0.99 | 0.99 | 0.99 | ||||||||||
Table 7: RF values of corresponding spots observed from the high performance thin layer chromatography profile of fractionated compounds of Aristolochia tagala kept in (a) iodine chamber, viewed in visible light and sprayed with Follin–Calcateau reagent, (b) sprayed with anisaldehyde, (c) sprayed with 1,1-diphenyl-2- picrylhydrazyl sprayed reagent
Tumor necrosis factor-α level in serum
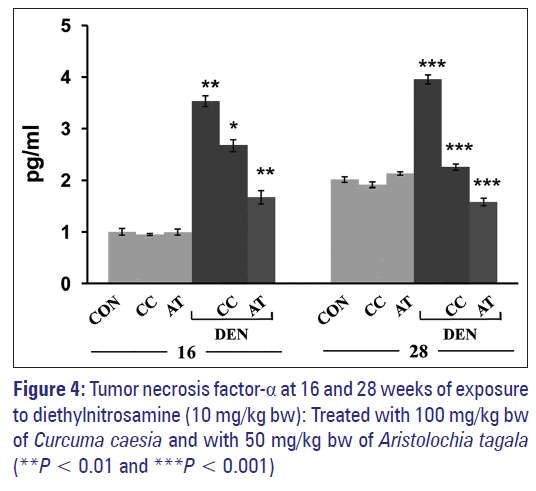
Exposure to DEN probably leads to a rapid accumulation of TNF-α in serum and was seen to increase significantly in comparison to control [Figure 4]. Treatment with CC (100 mg/kg bw) and AT (50 mg/kg bw) brought down the level of TNF-α [Figure 4] at 16 weeks. At 28 weeks period, the serum level of TNF-α in control was seen to increase in comparison to the control of 16 weeks [Figure 4]. DEN exposure caused a further elevation in the level of TNF-α. Treatment with both CC and AT significantly reduced the level of TNF-α in comparison to DEN-exposed groups [Figure 4]. Administration with the extracts of CC or AT alone did not cause any change in TNF-α in comparison to normal control at both 16 and 28 weeks [Figure 4].
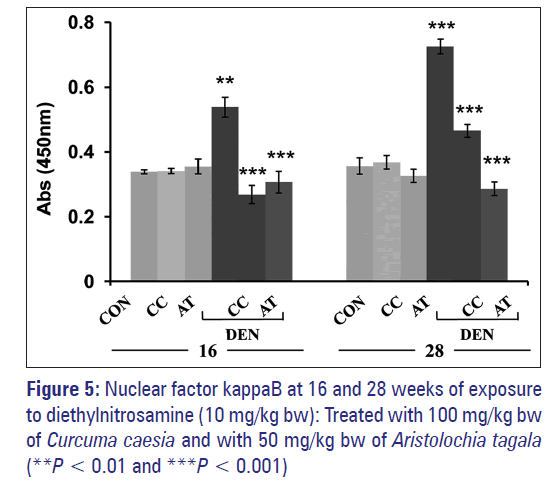
DNA binding activity of nuclear factor kappaB
Exposure to DEN probably leads to activation of NF-κB in nuclear extract of the liver as seen from the increase binding activity of NF-κB (p65) when compared to normal control [Figure 5]. Treatment with CC attenuated the effect of DEN where the NF-κB (p65) activity was seen to be almost similar to normal control. AT also caused a similar effect. At 28 weeks, a pattern similar to the one observed at 16 weeks was obtained [Figure 5]. DEN exposure caused a further increase in NF-κB (p65) activity in a nuclear extract of the liver. CC and AT attenuated the effect of DEN where the activity of NF-κB (p65) was very close to normal control. However, it appeared that AT was more effective than CC. Administration with the extract CC or AT alone did not cause any change in NF-κB (p65) activity in comparison to normal control at both 16 and 28 weeks.
Discussion
Medicinal plants contain several substances that could be used for therapeutic purposes. [20] They are precursors for the synthesis of useful drugs and are safe for human health. [21] Several substances from plants have shown anticarcinogenic effects by different mechanisms. Most of the phytochemicals such as glucosinolates and indoles, thiocyanates and isothiocyanates, phenols and coumarins can induce a faster elimination of carcinogenic compounds by inducing detoxifying Phase II enzymes. Ascorbate and phenols can block the formation of carcinogens such as nitrosamines. Flavonoids and carotenoids can disable the oxidative stress produced by some carcinogens acting as antioxidants. Moreover, carotenoids and other compounds can affect DNA synthesis and induce differentiation. [22] Phytochemical analysis conducted on the two plants extracts used in our study revealed the presence of constituents, which are known to exhibit medicinal as well as physiological activities. [23] Analysis of the extracts of these two plants, CC and AT revealed the presence of phytochemical compounds such as phenolics, tannins, flavonoids, terpenoids, and alkaloids [Table 1].
Polyphenols have also been found to possess a wide array of properties including chemopreventive, chemoprotective, chemosensitizing, radiosensitizing, and radioprotective activities. [24] Polyphenols are classified according to the nature of their carbon skeleton in: Phenolic acids, flavonoids, stilbenes, and lignans. Flavonoids are the most abundant polyphenol and can be classified into several different classes: Flavones (luteolin, apigenin), flavonols (quercetin), isoflavones (genistein and daidzein), anthocyanins (red fruids pigments), flavanols (catechins), proanthocyanidins (polymeric flavanols) and flavanones (hesperidin, narigin). [25] As in most of the cases of polyphenols, these flavonoids have been also shown to present cancer preventive properties. Phenolic acids such caffeic acid, ferulic acid, carnosic acid, etc., known to be a strong antioxidants, also possess antimutagenic and anticarcinogenic properties. [26,27] These compounds inhibit proliferation in leukemic cell lines such as HL-60 and U937 and in HeLa cells, which was associated with a reduction in tumor size. [28] In human colon carcinoma cells HT-29, flavonoids affect proliferation, differentiation and apoptosis with selectivity toward transformed cells. [29] Specific catechins have been proposed to optimize current chemotherapeutic protocols in leukemia. [30] The anthocyanin cyanidin-3-O-beta-glucopyranoside (Cy-g) is able to induce apoptosis in T-lymphoblastoid, whereas in HL-60 promyelocytic cells, Cy-g also induces differentiation to macrophage-like cells and granulocytes. [31] Furthermore, proanthocyanidins extracted from barley bran, prodelphinidin B-3, T1, T2, and T3, induce low levels of cell differentiation in HL-60 cells. [32] Our studies revealed the presence of high phenolic content in both CC and AT [Table 2], whereas high flavonoid content was found only in AT [Table 2].
Column chromatography was also performed to attempt to isolates the phytochemicals. Five fractions were obtained from CC and three from AT [Table 3a and b]. As these phytochemicals are known to exhibit antioxidant properties, the radical scavenging potential of the crude extract and fractions of CC and AT was measured by the reactivity toward the stable free radical DPPH. Our results revealed that both the extracts CC and AT showed antioxidant property by their ability to scavenge the free radical DPPH. CC crude extract as well as the fractions showed higher antioxidant property than AT [Table 4a and b and 5a and b, Figures 1 and 2]. The antioxidant property of CC may be contributed by phenolics, flavonoids as well as terpenoids, which were present in the plant as mentioned earlier, whereas the antioxidant property of AT may be contributed by only phenolics and flavonoids. The lower antioxidant property exhibited by AT could also be attributed to the compounds being in bound or polymerized forms, which can be released through hydrolysis. [33]
The bioactive compounds present in these two plants were identified by -HPTLC analysis. Our study showed the presence of phenolic compounds in CC and indicated that they may be catechol or hydroquinone [Figure 3a], terpenoids and also a number of other organic compounds. AT, on the other hand, showed the presence of flavonoids and indicated that they may be anthocynidin 3-glycosides and 6-hydroxylated flavonols. It also contained some flavones and chalcone glycosides [Figure 3b]. [18] Phenolic and flavonoids have been reported to have clear antioxidant properties in vitro. Since it was found that the plants contain the phytochemicals such as phenolics and flavonoids that could be responsible for its anticancer activity, quantitative analysis to estimate the total phenolics and total flavonoids was carried out by the two standardized and most commonly used methods. Many of their biological actions have been attributed to their antioxidant properties. As one of the significant mechanisms of induction of carcinogenesis is perceived to be through oxidative damage caused by increase ROS within the system and the induction of carcinogenesis by known carcinogens is also through the production of ROS that results in increased oxidative stress within the cells, it is, therefore, expected that the increase of cancer incidence could be reversed by removing avoidable load and/or sources of ROS. Thus, by quantifying the total phenolics and flavonoids, we can correlate that the anticancer properties exhibited by these two plants could perhaps be due to these phytochemical constituents present in these plants. The anticancer properties exhibited by AT may be due to phenolics and flavonoids whereas that of CC may be due to the other constituents present besides phenolics and flavonoids.
Exposure to DEN was seen to cause significant increase in TNF-α level in BALB/c mice. Treatment with the extracts CC or AT lowered the TNF-α at both 16 and 28 weeks [Figure 4]. This may be due to a reduction in DEN-induced inflammatory response by CC and AT extracts. Indeed, exposure to DEN was known to affect primarily liver tissue and cause hepatocyte death. [34-36] The inflammatory crosstalk between dying hepatocytes and myeloid cells is central to HCC development. One likely mechanism accounting for this crosstalk entails activation of Kupffer cells by products such as HMGB1 released by dying hepatocytes, a well-established proinflammatory mechanism. [37] The association of inflammation and cancer has been well recognized in many types of cancer and inflammation has been regarded as the “seventh hallmark of cancer.” [38] Accumulating evidence show that TNF-α is a key mediator of inflammation and cancer. [4,5] Administration of DEN was shown to cause rapid accumulation of TNF-α [39] and the release of this cytokine plays a crucial role in the carcinogenic process. [37] TNF-α receptors are expressed on both epithelial and stromal cells. In liver, tumor stromal cells, mainly macrophages or Kuffer cells generate the inflammatory cytokines TNF-α, which can directly facilitate cancer development by regulating the proliferation and survival of neoplastic cells. These cytokines attract and recruit more inflammatory cells such as dendritic cells and fibroblasts, which release other inflammatory cytokines such as IL-1 and IL-6. These further enhance the proliferation and survival of genetically altered cells. The interaction of hepatocytes with inflammatory cells results in an altered microenvironment that appears to be crucial both in the early and late stages of carcinogenesis. The role of TNF-α in the regulation of cell proliferation have been reported in various studies; for example, administration of TNF-α antibody prior to partial hepatectomy prevented hepatocyte proliferation and liver regeneration in rats. [40] Either TNF-αor TNF-α receptors-deficient mice have reduced susceptibility to chemically induced skin cancers and develop fewer experimental metastases. Inhibition of TNF-α also results in a marked reduction in tumor onset and tumor burden. [41]
TNF-α induced cell proliferation are mediated by the activation of NF-κB, PKCα and AP-1 dependent pathways. The evolutionarily conserved nuclear transcription factor NF-κB plays a crucial role in development, inflammation, immune responses, and cell survival. [42,43] NF-κB is critical for TNF-α induced tumor promotion. TNF-α induced signals lead to the activation of NF-κB allowing NF-κB translocation into the nucleus, and regulation of the transcription of target genes. [42,43] Several studies have provided evidence that NF-κB mediates pro-survival signals in the liver. The NF-κB-dependent signals involve the transcriptional activation of pro-survival genes. The first evidence of NF-κB in hepatocarcinogenesis using animal models comes from analyses of Mdr2-knockout mice, a model of inflammation-driven HCC. A study by Pikarsky et al. [44] demonstrates that hepatocytes adjacent to regions of inflammation in this model express high levels of nuclear NF-κB. It was also shown that NF-κB protects transformed hepatocytes from programmed cell death through the up-regulation of pro-survival genes. This and other data suggested that NF-κB is a tumor-promoting factor in hepatocytes. Our studies also shows that mice exposed to DEN in comparison to normal control mice showed an increase in NF-κB activity at 16 weeks [Figure 5]. This increase in NF-κB activity was further elevated at 28 weeks [Figure 5]. The increase in NF-κB activity correlates to increase levels of TNF-α. These results support that the secretion of pro-inflammatory cytokines-TNF-α from stromal cells is responsible for NF-κB activation, which stimulates compensatory proliferation of surviving hepatocytes with DNA damages. The NF-κB activity, however, was brought down to normal control when treated with the extracts CC or AT [Figure 5]. The extracts CC or AT might have reduced the inflammation thereby lowering TNF-α levels and subsequently reduce NF-κB activation. From our studies, it was also observed that at 16 weeks, CC was not able to reduce inflammation as efficiently as AT but was able to prevent NF-κB activation more efficiently [Figure 5]. Our results suggested that CC may be able to inhibit activation of NF-κB.
The presence of anthocynidin 3-glycosides, 6-hydroxylated flavonols, or chalcone glycosides was detected by HPTLC analysis only. Column or HPTLC were the methods chosen for constituent analysis as these are the standard basic methods, and our interpretations were based on these analyses. However, the detailed further analysis and structural identification using mass spectrophotometer and nuclear magnetic resonance analysis on fractions isolated by column or HPTLC will be carried out in near future once these facilities are available in our institute. This would perhaps enable us to overcome the limitations of the current study so that further confirmation and determination of the structure of the bioactive compounds of CC and AT can be achieved.
Conclusion
This study highlights the anticancer potential of CC and AT against DEN-induced HCC. It also suggested that CC and AT possess anti-inflammation, anti-proliferative and anti-cancer properties and that the active components of AT and CC may exert anticancer effects through the TNF-α-mediated NF-κB signaling.
Acknowledgment
The authors would like to thank UGC, Government of India for the financial support in the form of project grants to Prof. R. N. Sharan and Dr. L. Kma, and Rajiv Gandhi National Fellowship to Dr. K. L. H. Hadem to carry out the work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther 1992;53:127-66.
- Fiorani M, Cantoni O, Tasinato A, Boscoboinik D, Azzi A. Hydrogen peroxide-and fetal bovine serum-induced DNA synthesis in vascular smooth muscle cells: Positive and negative regulation by protein kinase C isoforms. Biochim Biophys Acta 1995;1269:98-104.
- Fausto N. Mouse liver tumorigenesis: Models, mechanisms, and relevance to human disease. Semin Liver Dis 1999;19:243-52.
- Sethi G, Sung B, Aggarwal BB. TNF: A master switch for inflammation to cancer. Front Biosci 2008;13:5094-107.
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9:361-71.
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003;10:45-65.
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 2001;414:308-13.
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001;414:313-7.
- McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med 1999;26:1034-53.
- Hadem KL, Sharan RN, Kma L. Inhibitory potential of methanolic extracts of Aristolochia tagala and Curcuma caesia on hepatocellular carcinoma induced by diethylnitrosamine in BALB/c mice. J Carcinog 2014;13:7.
- Cragg GM, Newman DJ, Weiss RB. Coral reefs, forests, and thermal vents: The worldwide exploration of nature for novel antitumor agents. Semin Oncol 1997;24:156-63.
- Harborne JB. Phytochemicals Methods. London: Chapman and Hall; 1973. p. 49-188.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. London: Bailliere Tindall; 1989. p. 45-50.
- Sofowra A. Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd.; 1993. p. 191-289.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144-53.
- Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 2000;72:35-42.
- Stock R, Rice CB. Chromatographic Methods. 3rd ed. New York: Halsted Press; 1974. p. 371-5.
- Harborne JB. Phytochemical Methods. 3rd ed. London: Chapman and Hall; 1998.
- Williams B, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Food Sci Technol Lebens Wiss Technol 1995;28:25-30.
- Syiem D, Syngai G, Khup PZ, Khongwir BS, Kharbuli B, Kayang H. Hypoglycemic effects of Potentilla fulgens L in normal and alloxan-induced diabetic mice. J Ethnopharmacol 2002;83:55-61.
- Wang J, Li J, Cao J, Jiang W. Antifungal activities of neem (Azadiractaindica) seed kernel extracts on post harvest diseases in fruits. Afr J Microbiol Res 2010;4:1100-4.
- Potter JD, Steinmetz K. Vegetables, fruit and phytoestrogens as preventive agents. IARC Sci Publ 1996;139:61-90.
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011;3:10-4.
- Jagetia G, Krishnan S, Aggarwal BB. Natural agents that can sensitize tumour cells to chemotherapy and radiation therapy. In: Bonavida B, editor. Sensitization of Cancer Cells for Chemo/Immuno/Radio-therapy. 1st ed. Totowa, NJ: Humana Press; 2008. p. 211-30.
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130 8S Suppl: 2073S-85S.
- Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A 1996;93:9090-5.
- Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer 2001;41:135-44.
- Jing Y, Yang J, Wang Y, Li H, Chen Y, Hu Q, et al. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor kappaB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med 2006;40:2183-97.
- Wenzel U, Kuntz S, Brendel MD, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res 2000;60:3823-31.
- Annabi B, Currie JC, Moghrabi A, Béliveau R. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leuk Res 2007;31:1277-84.
- Fimognari C, Berti F, Nüsse M, Cantelli-Forti G, Hrelia P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-beta-glucopyranoside. Biochem Pharmacol 2004;67:2047-56.
- Tamagawa K, Fukushima S, Kobori M, Shinmoto H, Tsushida T. Proanthocyanidins from barley bran potentiate retinoic acid-induced granulocytic and sodium butyrate-induced monocytic differentiation of HL60 cells. Biosci Biotechnol Biochem 1998;62:1483-7.
- Tsao R, Deng Z. Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B Analyt Technol Biomed Life Sci 2004;812:85-99.
- Cayama E, Tsuda H, Sarma DS, Farber E. Initiation of chemical carcinogenesis requires cell proliferation. Nature 1978;275:60-2.
- Lim IK. Spectrum of molecular changes during hepatocarcinogenesis induced by DEN and other chemicals in Fischer 344 male rats. Mech Ageing Dev 2002;123:1665-80.
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006;441:431-6.
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191-5.
- Mantovani A. Cancer: Inflaming metastasis. Nature 2009;457:36-7.
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005;121:977-90.
- Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, et al. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol 1992;263(4 Pt 1):G579-85.
- Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749-59.
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev 2004;18:2195-224.
- Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 2006;25:6800-16.
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6.