Magnetized solutions altered the absorbance of tramadol HCL: UV-spectrophotometer study
- *Corresponding Author:
Date of Received: 10-03-2010
Date of Modified: 10-04-2010
Date of Accepted: 22-04-2010
Available Online: 15-05-2010
Abstract
Magnetized water showed alterations in the electrolyte potential and vibration modes. This study aimed to investigate the effect of magnetic water and physiological solutions on the UV-spectra of tramadol HCl. Distilled water as well as full strength or diluted physiological solutions are magnetized by magnetic disc. Then tramadol HCl was dissolved in each magnetic and in non-magnetic solution to obtain the following final concentrations: 0, 25, 50, 100, and 200μg/mL. The pH of each solution and the absorbance (O.D.) was recorded at wavelength 271 nm. Magnetized water or physiological solutions showed alterations in pH as well as in the absorbance (O.D.) of tramadol HCl detected by ultraviolet spectra at λ271 nm. It concludes that magnetized physiological solutions adversely altered the stability of tramadol HCl.
https://marmarisinvestments.com
https://realestateinmarmaris.com
https://balloonsdocia.com
https://cappadociahotairballoon.org
Keywords
Magnetized solutions, Tramadol HCl
Introduction
There is no doubt that magnetic field, applied to water or cellular tissue alters their physiochemical properties. Pure water treated with high magnetic field in presence of oxygen resulted in a decrease in the contact angle of water on metals, an increase in the electrolyte potential of water and vibration modes [1]. Infrared and Roman spectroscopic investigation showed oxygen clathratelike hydrate and developed water network [2]. The intensity of fractions of fluorescence of water, water salts solutions and gel filtration of inorganic salt solutions is high when exposed to weak permanent and low frequency alternating magnetic fields [3]. Magnetic water showed scaling property against calcium ions [4] while the magnetic field affect the particulates but not solution of calcium carbonate [5].
At the cellular level magnetic field, as a result of interaction with cell membrane, induces changes in the magnitude of the ionic current density across the cell membrane and in the ionic concentration. It increases the uptake of water and this effect is utilized in speeding of seeds germination [6,7]. There is no report studying the effect of magnetic water in pharmaceuticals and clinical practice. Tramadol HCl is a central acting analgesic used in management of chronic pain [8] and recommended as 2nd line treatment for neuropathic pain [9]. This study aimed to investigate the effect of magnetic water and physiological solutions on the UV-spectra of tramadol HCl.
Materials and Methods
This study is conducted in department of pharmacology in cooperation with department of physiology/medical physics, college of medicine, university of Al-Musatnsiriya in Baghdad, Iraq during 2009. Distilled water is magnetized by magnetic disc (0.15 Tesla) composed from ferrous trioxide (Fe2O3) of ferrite magnet. The magnetic disc was immersed in glass container contained 500 mL of distilled water, or physiological solution; sodium chloride (0.9%), glucose saline (5%) or Ringer’s solution for one hour. In another series of experiments a 1/3 and 2/3 strength of sodium chloride (0.9%), and 0.2, 0,4, 0.6, 0.8 strength of glucose saline by diluting each sodium chloride (0.9%) or glucose saline (5% dextrose w/v and 0.9% sodium chloride) by corresponding volumes of distilled water. Then tramadol HCl was dissolved in each magnetic and in non-magnetic solution to obtain the following final concentrations: 0, 25, 50, 100, and 200 μg/mL. The pH of each solution and the absorbance (O.D.) was recorded at wavelength 271 nm. The results are expressed as absolute number and a simple correlation test with linear regression was applied for calculation the absorbance (O.D.) of corresponding forecast value of 50 μg/mL tramadol HCl.
Results
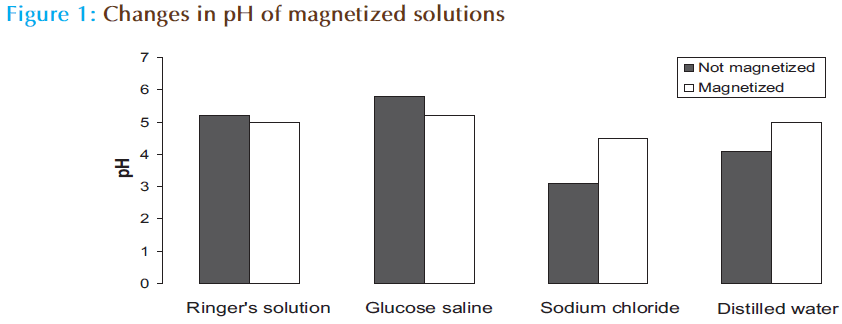
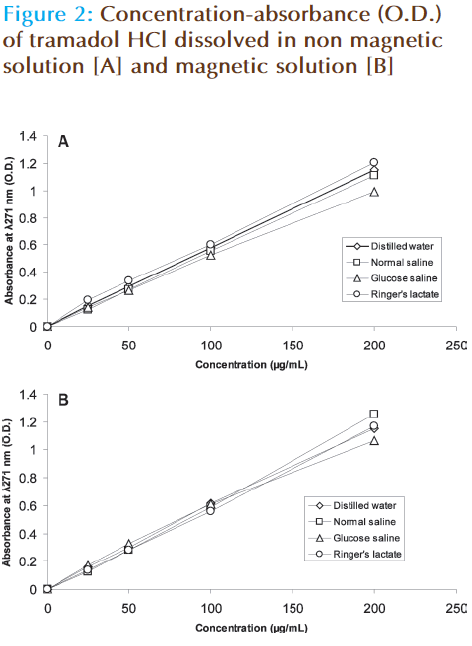
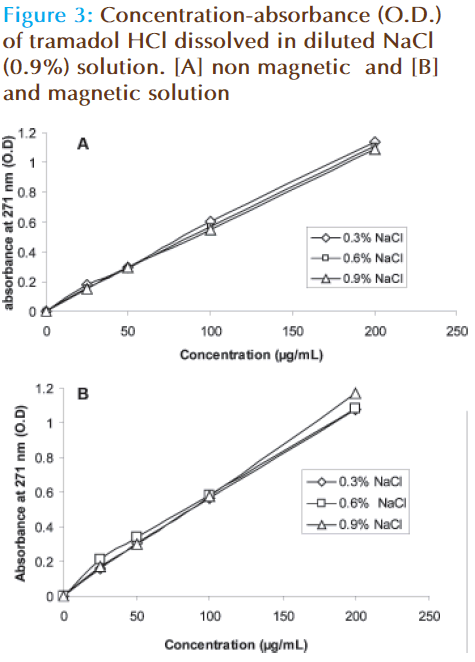
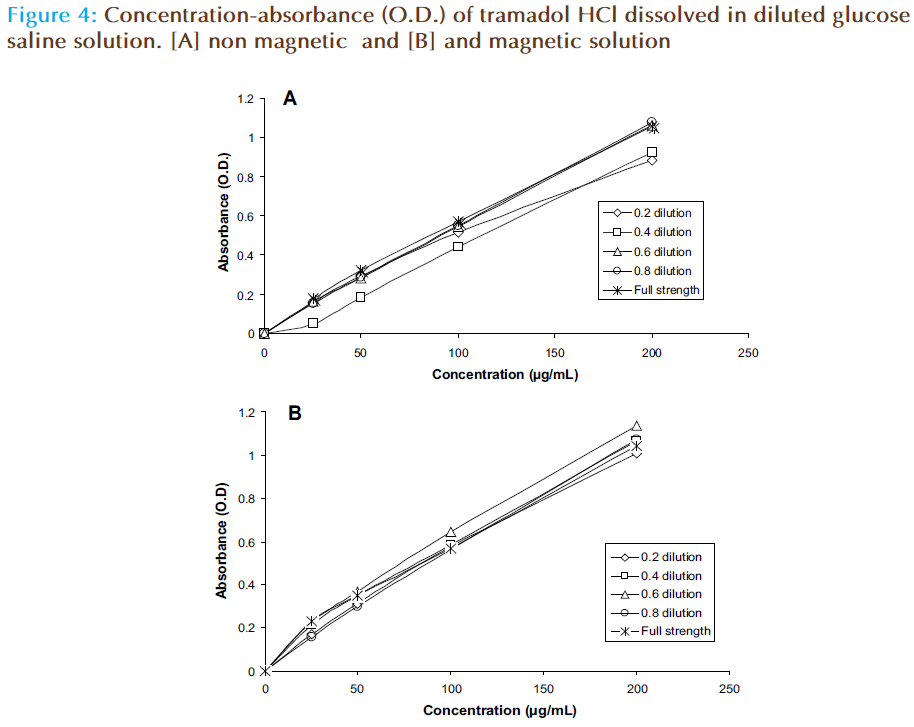
The pH of distilled water or sodium chloride (0.9%) was reduced while that of glucose saline (5%) or Ringer’s solution is increased (Figure 1). The pH of glucose saline is increased from 3.1 to 4.5. The absorbance (O.D.) – concentration curve of tramadol HCl when it dissolved in magnetic solution is differed from that of nonmagnetic solution (Figure 2). The forecast value of absorbance for corresponding concentration (50 μg/mL) of tramadol HCl dissolved in magnetic distilled water, sodium chloride (0.9%) or glucose saline was higher than corresponding values of non magnetized solutions. The absorbance (O.D.) is decreased from 0.3208 to 0.2838 for 50 μg/mL tramadol HCl dissolved in Ringer’s solution (Table 1). The absorbance concentration curves of tramadol in magnetic sodium chloride solutions of different dilutions were also differed from corresponding non magnetic solutions (Figure 3). Table 2 showed that higher forecast value of absorbance corresponding to 50 μg/mL tramadol HCl dissolved in 2/3 strength of sodium chloride (0.9%). This observation was also observed when the glucose saline is diluted to 3/5 strength (Figure 4 and Table 2).
| Solution | Correlation factor (r) | Intersept | Slope | Calculated O.D. for 50 μg/mL |
|---|---|---|---|---|
| Distilled water | ||||
| Non magnetized | 0.99998 | 0.0036 | 0.0057 | 0.2912 |
| Magnetized | 0.99941 | 0.0137 | 0.0057 | 0.3019 |
| Sodium chloride (0.9%) | ||||
| Non magnetized | 0.99988 | -0.0058 | 0.0055 | 0.2729 |
| Magnetized | 0.99923 | -0.0252 | 0.0063 | 0.2918 |
| Glucose saline | ||||
| Non magnetized | 0.99953 | 0.0135 | 0.0049 | 0.2610 |
| Magnetized | 0.99625 | 0.0405 | 0.0052 | 0.3043 |
| Ringer lactate | ||||
| Non magnetized | 0.99905 | 0.0252 | 0.0059 | 0.3208 |
| Magnetized | 0.99969 | -0.0083 | 0.0058 | 0.2838 |
Table 1: The effect of magnetic solution on the statistical results of absorbance (O.D.) - concentration correlation of tramadol HCl
| Solution | Correlation factor (r) | Intersept (a) | Slope (b) | Calculated O.D. for 50 μg/mL |
|---|---|---|---|---|
| 0.9% Sodium chloride solution(1/3 strength) | ||||
| Non magnetized | 0.99927 | 0.0189 | 0.0056 | 0.3007 |
| Magnetized | 0.99929 | 0.0217 | 0.0053 | 0.2879 |
| 0.9% Sodium chloride solution (2/3 strength) | ||||
| Non magnetized | 0.99987 | 0.0103 | 0.0055 | 0.2866 |
| Magnetize | 0.99646 | 0.0525 | 0.0052 | 0.2983 |
| Sodium chloride solution (0.9%) | ||||
| Non magnetized | 0.99988 | -0.0058 | 0.0055 | 0.2729 |
| Magnetized | 0.99923 | -0.0252 | 0.0063 | 0.2918 |
| Glucose saline solution (1/5 strength) | ||||
| Non magnetized | 0.99450 | 0.0461 | 0.0043 | 0.2625 |
| Magnetized | 0.99628 | 0.0417 | 0.0050 | 0.2893 |
| Glucose saline solution (2/5 strength) | ||||
| Non magnetized | 0.99751 | -0.0388 | 0.0048 | 0.1992 |
| Magnetized | 0.99535 | 0.0587 | 0.0051 | 0.3147 |
| Glucose saline solution (3/5 strength) | ||||
| Non magnetized | 0.99953 | 0.0182 | 0.0052 | 0.2799 |
| Magnetized | 0.99494 | 0.064 | 0.0055 | 0.3384 |
| Glucose saline solution (4/5 strength) | ||||
| Non magnetized | 0.99780 | 0.0129 | 0.0053 | 0.2796 |
| Magnetized | ||||
| 0.99927 | 0.0222 | 0.0053 | 0.2868 | |
| Glucose saline solution | ||||
| Non magnetized | 0.99953 | 0.0135 | 0.0049 | 0.2610 |
| Magnetized | 0.99625 | 0.0405 | 0.0052 | 0.3043 |
Table 2: The effect of magnetic diluted solution on the statistical results of absorbance (O.D.) - concentration correlation of tramadol HCl
Discussion
Tramadol HCl dissolved in magnetized distilled water or physiological solutions showed different peak magnitude in UV-spectra compared with non magnetized solutions. This is the first report that shows the effect of magnetized solution on the pharmaceutical dissolution. The results of this work agree the results of Jin et al study which showed the wavelength spectra of absorption of some solvents and solutions did not change but the strength of the absorption of all magnetized compounds changed [10]. Previous studies showed that ozonation of water removed 80% of caffeine, pharmaceutical and endocrine disruptors [11]. Also solar Photo-Fenton of distilled water decreased sulfamethoxazole solution toxicity from 85% to 20% [12]. The explanation of different absorbance magnitude of tramadol HCl at wavelength λ 271 nm when it dissolved in magnetic water is related to the generation of exogenous hydrogen peroxide which interfered with tramadol HCl absorbance. The generation of exogenous hydrogen peroxide in magnetized solution was previously proved [13]. The clinical significance of the present study relied on: first, tramadol HCl is a central analgesic compound and the action potentials of neurons are significantly enhanced under pulsed magnetic field [14]. Second, it was reported that the application of low magnetic field gradient lead to pull molecules and living cells to magnetic particle [15] , therefore, the molecule of tramadol HCl may be trapped in the molecules of magnetized solutions. Third, pulsed electrical field reduced the cell wall permeability [13], therefore, the availability of intracellular tramadol HCl dissolved in magnetized solutions will be reduced. On the other hand, magnetized solutions reduce the calcium influx and inhibit sodium-potassium ATP-ase pump leading to depress the myocardial contractility [16,17]. This effect will add a further adverse reaction when magnetized solution of tramadol HCl is used. The study limitations include application of pulsed magnetized field to the physiological solutions and the stability testing of magnetized solutions. It concludes that magnetized physiological solutions altered the stability of tramadol HCl.
References
- Otsuka I , Ozeki S. Does magnetic treatment of water changes its properties? J Phys Chem B 2006; 110(4): 1509-12.
- Ozeki S, Otsuka S. Transient oxygen clathrate-like hydrate and water networks induced by magnetic fields. J Phys Chem B 110(41): 20067-72.
- Iablokova EV, Novikova VV, Fesenko EE. Effect of weak magnetic fields on fluorescence of water and water-salt solutions. Partial characterization of fluorescing fractions. Biofizika 2007; 52(2): 197-204.
- Gabrielli C, Jaouhari R, Maurin G, et al. Magnetic water treatment for scale prevention. Water Res 2001; 35(13): 3249-59.
- Kney AD, Parsons SA. A spectrophotometer-based study of magnetic water treatment: assessment of ionic vs. surface mechanisms. Water Res 2006; 40(3): 517-24.
- Reina FG, Pascual LA. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: experimental results. Bioelectromagnetics 2001; 22(8): 589-95.
- Reina FG, Pascual LA, Fundora IA. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: experimental results. Bioelectromagnetics 2001; 22(8): 596-602.
- Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry 2009; 31(3): 206-19.
- O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009; 122(10 Suppl) S22-32.
- Jin Z, Xiao L, Wang Y, et al. Application of UV spectrophotometry in the detection of magnetization effect. Guang Pu Xue Yu Guang Pu Fen Xi 2000; 20(3): 418-19.
- Broséus R, Vincent S, Aboulfadl K, et al. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res 2009; 43(18): 4707-17.
- Trovó AG, Nougueira RFP, Agüera A, et al. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 2009; 43(16): 3922-31.
- Galindo F-G, Thomas Vernier P, Dejmek P, et al. Pulsed electric field reduces the permeability of a potato cell wall. Bioelectromagnetics 2008; 29(4): 296-301.
- Ahmed Z, Wieraszko A. The influence of pulsed magnetic fields (PMFs) on nonsynapteic potentials recorded from the central and peripheral nervous system in vitro. Bioelectromagnetics 2009; 30(8): 621-30.
- Xia N, Hunt TP, Mayers BT, et al. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed Microdevices 2006 Sept 25.
- Ayrapetyan G, Papanyan A, Hayrapetyan H, et al. Metabolic pathway of magnetized fluid-induced relaxation effects on heart muscle. Bioelectromagnetics 2005; 26(8): 624-30.
- Ayrapetyan G, Dadasyan E, Hayrapetyan H, et al. Exogenous hydrogen peroxide as a possible messenger for the stimulation of magnetized physiological solution on heart contractility. Bioelectromagnetics 2008; 29(7): 549-58.