Isolation of anticancer drug TAXOL from Pestalotiopsis breviseta with apoptosis and B-Cell lymphoma protein docking studies
- *Corresponding Author:
- Dr. G. Kathiravan
Department of Biotechnology, Vels University, Pallavaram, Chennai - 600 117, Tamil Nadu, India.
E-mail: gkathir72@gmail.com
Abstract
Background: Extraction and investigation of TAXOL from Pestalotiopsis breviseta (Sacc.) using protein docking, which is a computational technique that samples conformations of small molecules in protein-binding sites. Scoring functions are used to assess which of these conformations best complements the protein binding site and active site prediction. Materials and Methods: Coelomycetous fungi P. breviseta (Sacc.) Steyaert was screened for the production of TAXOL, an anticancer drug. Results: TAXOL production was conô€ÂÂÂÂirmed by the following methods: Ultraviolet (UV) spectroscopic analysis, Infrared analysis, High performance liquid chromatography analysis (HPLC), and Liquid chromatography mass spectrum (LC-MASS). TAXOL produced by the fungi was compared with authentic TAXOL, and protein docking studies were performed. Conclusion: The BCL2 protein of human origin showed a higher afô€ÂÂÂÂinity toward the compound paclitaxel. It has the binding energy value of −13.0061 (KJ/Mol) with four hydrogen bonds.
Keywords
Pesalotiopsis breviseta, BCL protein, TAXOL, protein docking, isolation
Introduction
TAXOL is the commercial trade name used by Bristal-Myers Squibb for this compound, while paclitaxel is the chemical name. TAXOL has a novel and complex chemical structure along with a chemically similar analog, Taxotene (Docetaxel) [1] and has a unique antitumor mechanism of action. TAXOL promotes microtubule assembly and stabilizes microtubule polymers, thereby blocking cell replication.[2] The drugs are believed to block cell-cycle progression during mitosis by binding to and stabilizing microtubules.[3] During cell division, TAXOL interferes with the development of the microtubules needed for cell duplication, thus inhibiting the faster growing tumor cells. This mechanism is different from other anticancer agents that work by interfering with the DNA of the tumor cells. Clinical development of TAXOL progressed slowly because of the extremely small amount of drug obtained from the crude bark extract, and its poor water solubility. TAXOL was approved for the treatment of ovarian cancer by the Food and Drug Administration (FDA) in 1992 and approved for treatment of breast cancer in 1992. Clinical use of TAXOL has increased steadily since then and today it is used not only for treatment of ovarian and breast cancers, but also for treatment of lung cancer, squamous cancers of the head and neck, and various other cancers. Pacific yew grows slowly in the understory of natural stands, but under intensive nursery culture, can grow quite quickly. Pacific yew generally lives for 200-300 years with some species living for 400 years or more. TAXOL content can vary and is found in small quantities (0.001% to 0.01% of the dry bark weight). It is generally considered to take 3-10 trees per patient. Based on the data from the US Forest Service from 1992, 36000 trees are required to provide 327200 kg of bark (about 9 kg/ tree) from which 24 kg of TAXOL can be extracted (about0.66 g/tree). Hence, there is an urgent to search for the alternative sources for this promising anticancer drug.
The plant cell culturing of Taxus species is considered to be a promising method to obtain TAXOL and related taxane compounds.[4] Investigated the production of TAXOL using free and immobilized cells of Taxus cuspidatausing perfusion culture reactors, and reported a continuous TAXOL production rate of 0.3 mg/g dry cell weight (DCW) per day for 40 days. An exciting development in 1993 was that TAXOL could be produced by the fungus Taxomyces andreanae [4] isolated from the inner bark of a yew tree growing in North- Western Montana, appeared to produce TAXOL and other taxanes in denovo fashion when grown in semi-synthetic liquid media. The yield was unfortunately very low (24-50 ng/l). The presence of various microorganisms from the bark of the yew tree was found to be capable of producing TAXOL. Pestalotiopsis microspora, a coelomycetous endophyte in Taxus brevefolia under cultural condition was observed to produce TAXOL [5] in an amount considerably more than that of Taxus sp. There are at least 12 distinct enzymatic reactions involved in TAXOL biosynthesis.[6,7] The genetic manipulation of fungi could be achieved more easily than that of plants, and it may be possible to improve the production significantly with the help of genetic engineering. Keeping this in mind, there is an urgent need to search for the alternative sources of this promising anticancer drug. The present study deals with the screening of selected endophytic fungi for the production of TAXOL. In vitro TAXOL production, by Pestalotiopsis breviseta – A first report was made by.[8] TAXOL production by the coelomycetous fungus, P. breviseta was confirmed by Ultraviolet (UV), Infrared (IR), High performance liquid chromatography (HPLC), Nuclear magnetic resonance (NMR), and Liquid chromatography mass spectrum (LCMASS) spectroscopy. The TAXOL produced was identical with authentic TAXOL. Coelomycetous fungi were screened for the production of TAXOL was confirmed by UV, IR, HPLC, NMR and LC-MASS spectroscopy. TAXOL was identical with authentic TAXOL[9,10] reported. Effect of TAXOL from Pestalotiopsis mangiferae on A549 cells – in vitro study of antiproliferative activity in human Non-small cell lung cancer A549 cells.
Materials and Methods
Extraction of TAXOL
The extraction procedure was carried out by the method of, [5] the test fungus selected in this study was grown in 2-liter Erlenmeyer flasks containing 500 ml of MID medium supplemented with 1 g soytone/l.[11] The test fungus was inoculated into the medium and incubated for 21 days at 26 ± 1°C. After completion of the incubation period, the culture was harvested and the culture filtrate was passed through four-layered cheesecloth. In order to avoid fatty acid contamination, 0.25 g of NaCO3 was added to the filtrate and extracted with two equal volumes of solvent Dichloromethane. The organic phase was collected and evaporated to dryness under reduced pressure at 35°C. The dry solid residue was re-dissolved in methanol and placed on a 1.5 × 30-cm column of silica gel (Baker 40 μ). Elution of the column was performed in a stepwise manner starting with 70 mL of 100% methylene chloride followed by mixtures of organic solvents at different proportions. The fractions thus obtained were collected, evaporated to dryness, and subjected to thin layer chromatography (TLC). The presence of TAXOL in the fungal sample was an analyzed by TLC, UV absorptions spectrophotometry, IR spectroscopic analysis, HPLC analysis, and MASS spectroscopic analysis.
Column chromatography (CC)
A 1.5 × 30-cm column of silica gels loaded was with sample dissolved in methylene chloride. Elution of the sample was done in a stepwise manner with solvent system as follows 70 mL 100% ethylene chloride, 20:1 v:v methylene chloride:ethylacetate, 10:1 v:v methylene chloride:ethylacetate, 6:1 v:v methylene chloride:ethylacetate, 3:1 v:v methylene chloride:ethyl acetate,1:1 v:v methylene chloride:ethylacetate. Fractions having same mobility as the authentic TAXOL were combined and evaporated to dryness. The residue was subjected to TLC.
Thin layer chromatography
TLC was done on 1 mm and 0.25 mm on Merck precoatedsilicagel plates. The plates were developed successively in each of the following solvent systems as follows A – chloroform:methanol 7:1 v:v, B–chloroform: acetonitrile 7:3 v:v, C–ethyl acetate: propanol 95:5 v:v, D–methylenechloride: tetrahydro furan 6:2 v:v, E–methylene chloride:methanol:dimethy lformamide 90:9:1 v:v:v. TAXOL was detected with 1% w/v vanillin/sulphuric acid reagent after gentle heating.[12] It appeared as a bluish spot fading to dark grey after 24 h.
Ultraviolet absorption spectral analysis
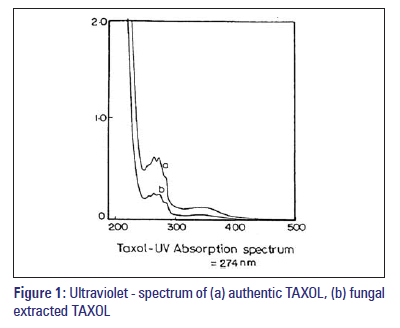
The sample containing TAXOL was analysed spectroscopically for further confirmation of TAXOL. After chromatography, the area of the plate containing putative TAXOL was carefully removed by scrapping off the silica at the appropriate Rf and exhaustively eluting it with methanol. The UV spectral analysis of fungal sample was superimposed on that of authentic TAXOL at 273 nm.
High Pressure Liquid Chromatography analysis
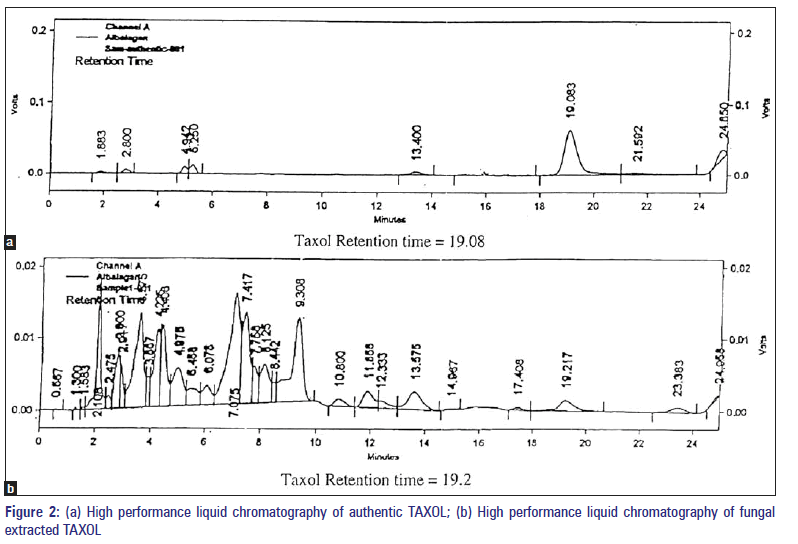
TAXOL was analyzed by HPLC (Shimatzu 9A model) using a reverse phase C18 column with a UV detector. A C18 column was used for determining the behavior of the fungal compound by HPLC. A total volume of 20 μl of the sample was injected each time and detected at 232 nm. The mobile phase was methanol:acetonitrile:water (25:35:40, by Vol.) at 1.0 ml/ min. TAXOL was quantified by comparing the peak area of the samples with that of the TAXOL standard using the formula:

Infrared spectroscopic analysis
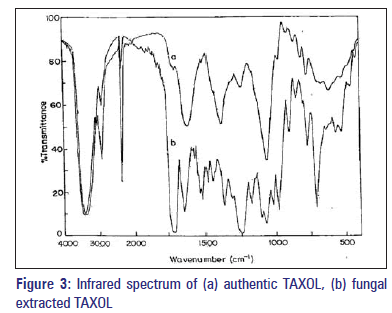
The IR spectra of the compound were recorded on Shimadzu FT IR 8000 series instrument. The purified TAXOL was ground with IR grade potassium bromide (KBr) (1:10) pressed in to discs under vacuum using spectra lab pelletizer and compared with authentic TAXOL. The IR spectrum was recorded in the region 4000-500/cm.
Liquid chromatography mass spectrum analysis
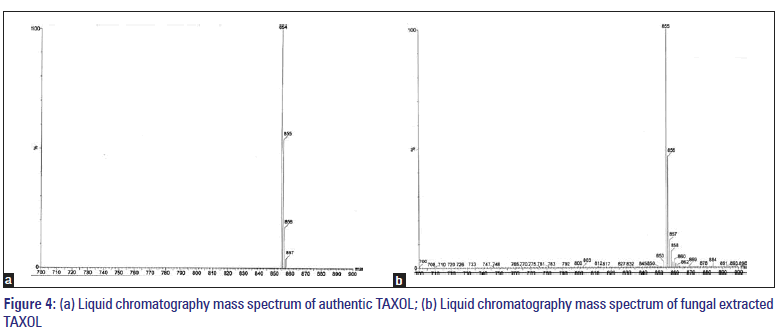
Samples selected for fractionation, were the fractions of the above two fungi. They were checked for confirming the presence of TAXOL using mass spectroscopy. For electron spray method, the sample was dissolved in methanol:water:aceticacid (50:50:1 by 5.Vol.). The fungal compound produced an identical LC-MASS spectrum as authentic paclitaxel. Characteristically, authentic TAXOL yielded both an (M + H) +peak at 855 and an (M + Na) + peak at 856.[13] By comparison, fungal TAXOL also yielded an (M + H) +peak at 855 and an (M + Na) + peak at 856.
Results
In the present study, the test fungus P. breviseta, a Coelomycete, was tested for TAXOL production and enhancement of TAXOL production by different elicitors like biotic and abiotic. Semi-synthetic media were used for the growth of the fungi.[14] Studied the induction by methyl jasmonate and salicylic acid of TAXOL and relevant taxane biosynthesis in suspension cultures of Taxuschinensis var. mairei. The elicitor molecules binding with hypothetical receptor molecules for methyl jasmonateinducing TAXOL biosynthesis is about 75% lower than that for salicylic acid. P. microspore is an endophytic fungi associated with many plants including Taxuswallachiana, commonly produces TAXOL in liquid culture. Defining culture amendments to optimize TAXOL production by P. microspore is a critical step toward the realization of fungal TAXOL for treating human cancers. The lowering of phosphate and the addition of Sodium benzoate in the medium increased TAXOL production. TAXOL was extracted using dichloromethane, the solvent was then removed by evaporation under vacuum and the residue was re-dissolved in methanol. Sample solution was analyzed by TLC, UV, HPLC and IR spectroscopy.[8-10]
The fungal extracts were subjected to TLC on a 0.25-mm (10 × 20 cm) silica gel plate developed in different solvent system with authentic TAXOL (Sigma, Cat. No. T-7402) as standard.[12] They showed identical UV characteristics and reacted positively with Vanillin/H2SO4 spray reagent, yielding a blue spot which turned grey after 12-24 h. They had Rf values identical to that of authentic TAXOL. After chromatography, the area of the plate containing putative TAXOL was carefully removed by scrapping off the silica at the appropriate Rf and exhaustively eluting it with methanol. The UV spectral analysis of fungal samples was superimposed on that of authentic TAXOL with two maxima at 273 nm and 235 nm [Figure 1a and b].
The presence of TAXOL was further confirmed using HPLC. TAXOL was analysed by HPLC (Shimatzu 9A model) using a reverse phase C18 column with a UV detector. A total volume 20 μl of sample was injected each time and detected at 232 nm. The amount of TAXOL produced by the fungus was quantified by comparing the peak area of the samples [Figure 2a and b] with that of the TAXOL standard using the formula. Further convincing evidence for the identity of TAXOL was obtained by HPLC.
The presence of TAXOL was further confirmed using IR analysis (IR of the compounds isolated from the fungal samples [Figure 3a and b]. The appearances of bands convincingly illustrate the identical feature of the extracted samples the authentic TAXOL. IR analysis showed that the presence of alcoholic O-group in the present compound is evident by its OH stretch at 3448/cm, the aliphatic CHstretch at 2931/cm. The C=O stretch positioned 1724/cm, whereas the amide C=O stretch is shifted to lower value at 1652/cm. The instance peak at 1247.16/cm is due to COO stretch. The alkyl C-O stretch of ester is observed at 1072/ cm. The peak at 707/cm is due to aromatic C, H bond. The overtone is at 2362.64/cm. In the extracted sample, though the intensity of the bands is very much diminished in the finger printing region, appearance of overtone 2362/cm convincingly illustrates the identical nature of the extracted sample with authentic TAXOL. The fungal compound produced an identical LC-MASS spectrum as authentic paclitaxel. Characteristically, authentic TAXOL yielded both an (M + H) + peak at 855 and an (M + Na) + peak at 856. By comparison, fungal TAXOL also yielded an (M + H) + peak at 855 and an (M +Na) + peak at 856 [Figure 4a and b].
Apoptosis studies
Hoechst 33258 staining–Cells were cultured under normal conditions in the presence or absence of Hep2 culture medium for 24 h. Then, cells were washed with Phosphate Buffer Slain and fixed with 10% neutral buffered formalin. After fixation, cells were stained with 1 μg/ml of Hoechst 33258 (Molecular Probes, Eugene, Oregon) for 10 min at 37°C in the dark. Cells were washed twice with PBS and were analyzed under a fluorescent microscope (Olympus, Melville, New York). Apoptotic cells were identified by nuclear condensation, formation of membrane blebs and apoptotic bodies. The mean percentage of apoptotic cells was estimated by counting three random fields induplicate wells per group and today, it is used not only for the treatment of ovarian and breast cancers, but also for the treatment of lung cancer, squamous cancers of the head and neck, and various other cancers.[13] Pacific yew grows slowly in the understory of natural stands but under intensive nursery culture, it can grow quite quickly. Pacific yew generally lives for 200-300 years with some species living for 400 years or more. TAXOL content can vary and is found in small quantities (0.001% to 0.01% of the dry bark weight). It is generally considered that 3-10 trees are required per patient. Based on the data from the US Forest Service from 1992, 36000 trees are required to provide 327200 kg of bark (about 9 kg/tree) from which 24 kg of TAXOL can be extracted (about 0.66 g/tree). Hence, there is an urgent need to search for the alternative sources for this promising anticancer drug.
The plant cell culturing of Taxus species is considered to be a capable method to find TAXOL and related taxane compounds.[15] investigated the production of TAXOL using free and immobilized cells of T. cuspidate using perfusion culture reactors, continuous TAXOL production rate of 0.3 mg/g DCW per day for 40 days. An exciting development in 1993 was that TAXOL could be produced by the fungus T. andreanae [4] isolated from the inner bark of a yew tree growing in North-Western Montana, appeared to produce TAXOL and other taxanes in denovo fashion when grown in semi-synthetic liquid media. The yield was unfortunately very low (24-50 ng/l). The presence of various microorganisms from the bark of the yew tree was found to be capable of producing TAXOL. P. microspora, a coelomycetous endophyte in T. brevefolia under cultural condition was observed to produce TAXOL [5] in considerable amount more than the Taxus sp. There are at least 12 distinct enzymatic reactions involved in TAXOL biosynthesis.[6,7,16,17] The genetic manipulation of fungi could be achieved more easily than that of plants, and it may be possible to improve the production significantly with the help of genetic engineering. Keeping this in mind there is an urgent to search for the alternative sources for this promising anticancer drug. The present study deals with the screening of selected endophytic fungi for the production of TAXOL. In vitro TAXOL production, by P. breviseta – A first report was made by [8] Coelomycetous fungi P. breviseta. TAXOL production was confirmed by UV, IR, HPLC, NMR and LCMASS spectroscopy. TAXOL was identical with authentic TAXOL. Two different Coelomycetous fungi were screened for the production of TAXOL was confirmed by UV, IR, HPLC, NMR and LC-MASS spectroscopy. TAXOL was identical with authentic TAXOL [9,10] reported. Effect of TAXOL from P. mangiferae on A549 cells –in vitro study of antiproliferative activity in human non small cell lung cancer A549 cells.
Active site prediction
The active site of the BCL2 protein was found through Computed Atlas of Surface Topography of Proteins CASTp. These include pockets located on protein surfaces and avoids buried in the interior of proteins. CASTp includes a graphical user interface, flexible interactive visualization, as well as onthe- fly calculation for user-uploaded structures.[18]
Docking the Drug against the protein
Docking is a computational technique that samples conformations of small molecules in protein binding sites. Scoring functions are used to assess which of these conformations best complements the protein binding site.[19] The inhibitor and target protein was geometrically optimized and docked using docking engine Argus Dock (http://www. arguslab.com/).
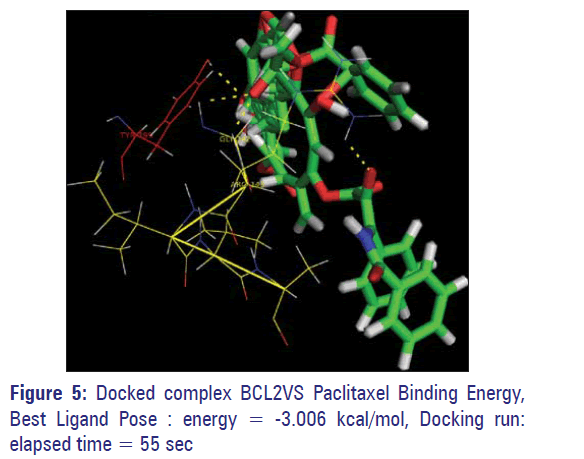
The BCL2 protein of human origin showed a higher affinity toward the compound Paclitaxel. It has the binding energy value of−13.0061 (KJ/Mol) with four hydrogen bonds [Table 1]. The two hydrogen bonds were formed between the Paclitaxel and TYROSIN 199 with a distance of 2.2A and 2.3A. The third and fourth hydrogen bond is formed with GLYSINE 142 and ARGININE143 of the active site residue with a of distance of 2.5A and 2.3A respectively [Figure 5].
| Residue | Atom | Taxol | Distance |
|---|---|---|---|
| TYR199 | H | O | 2.2 |
| GLY 142 | H | O | 2.5 |
| RG143 | H | O | 2.3 |
Tyrosine, Glysine Arginine
Table 1: Hydrogen bond interaction.
Source of Support: Nil, Conflfl ict of Interest: No.
References
- Guenard D, Gueritte VF, Potier P. Taxol, taxoltere: Discovery, chemistry, and structure-Activity relationships. Acc Chem Res 1993;26:160-7.
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980;77:1561-65.
- Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, et al. Total synthesis of taxol. Nature 1994;367:630-34.
- Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993;260:214-6.
- Strobel G, Yang X, Sears J, Kramer R, Sidhu RS, Hess WM. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 1996;142:435-40.
- Croteau R, Hefner J, Hezari M, Lewis NG. In: Flores HE, Gustine DL editors. Phytochemicals and Health. (Am. Soc. Plant Physiol. Rockville, MD), 1995. p. 94-104.
- Floss HG, Mocek U. Biosynthesis of taxol. In: Suffness M, editor. Boca Raton: Taxol: Sci. Appl., CRC Press USA; 1995. p. 191-208.
- Kathiravan G, Sri Raman V. In vitro TAXOL production, by Pestalotiopsis breviseta ? A first report. Fitoterapia 2010;81:557-4.
- Kathiravan G, Muthumary J. Extraction of taxol, an anticancer drug by coelomycetous fungi Pestalotiopsisversi color and Phyllostictamurrayicola Mycologia Balcanica. J Mycologia Balcanica 2009;6:69-4.
- Kathiravan G, Sripathi M. Effect of taxol from Pestalotiopsis mangiferae on A549 cells - In vitro study. J Basic Clin Pharma 2010;1:1-9.
- Pinkerton F, Strobel G. Serinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchari. Proc Natl Acad Sci U S A 1976;73:4007-11.
- Cardellina, JH. HPLC separation of taxol and cephalomannine. J Chrom 1991;14:659.
- Yeung TK, Germond C, Chen X, Wang Z. The mode of action of taxol: Apoptosis at low concentration and necrosis at high concentration. Biochem Biophys Res Commun 1999;263:398-404.
- Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X. Taxol from fungal endophytes and the issue of biodiversity. J Int Microb Biotechnol 1990;179:417-23.
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971;93:2325-7.
- Kumaran RS, Muthumary J, Hur BK. Production of taxol from Phyllostictaspinarum, an endophytic fungus of Cupressussp. Eng Life Sci 2008a;8:438-46.
- Kumaran RS, Muthumary J, Hur BK. Taxol from Phyllostictacitricarpa, a leaf spot fungus of the angiosperm Citrus medica. J Biosci Bioeng 2008;106:103-6.
- Binkowski TA, Naghibzadeh S, Liang J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res 2003;31: 3352-5.
- Warren GL, Andrews CW, Capelli AM, Clarke B, LaLonde J, Lambert MH, et al. A critical assessment of docking programs and scoring functions. J Med Chem 2006;49:5912-31.