Improving monitoring and reporting of adverse drug reactions (ADRs) in HIV positive patients on antiretroviral therapy (ART) in Nigeria
- *Corresponding Author:
Date of Received: 31-03-2012
Date of Accepted: 03-05-2012
Available Online: 15-05-2012
Abstract
Under-reporting of ADR may be associated with poor knowledge, attitudes and practices to pharmacovigilance. This study evaluated knowledge, attitudes and practices of healthcare professionals about ADR monitoring and reporting following interventions. This longitudinal study included 36 healthcare professionals participating in ART program in a tertiary hospital. Interventions included group training on pharmacovigilance (PV) and provision of ADR reporting forms amongst others. Assessments were conducted at months 0 and 6 post-interventions using study-specific Likerttype instruments. Mean attitude scores above midpoint of 3.6 on 5-point scale were regarded as positive and below as negative. P<0.05 used to determine statistical significance. Mean age of participants was 36.6 (95%CI, 34.5–38.7) years; 61.1% males; 44.4% doctors, 13.9% pharmacists, 19.4% nurses, 8.3% laboratory scientists, 8.3% record officers and 5.6% welfare officers. None had received training on PV previously. Mean knowledge test score increased from 53.6% (95%CI, 44.6–63.6) at pre-intervention to 77.1% (95%CI, 72.8–81.4) at post-intervention with a mean change of 146.9% (95%CI, 60.5–233.3; p=0.000). Mean rated attitude scores increased from 3.6 (95%CI, 3.4–3.8) at pre-intervention to 4.2 (95%CI, 4.0–4.4) at post-intervention; the difference was statistically significant (p=0.000). 75.8% reported that ADR reporting forms were not readily available at pre-intervention compared to 18.2% at postintervention; 15.2% had reported ADR previously at pre-intervention compared to 69.7% at post-intervention; 12.1% reported providing information regarding ADRs and its management always at pre-intervention compared to 45.5% at post-intervention; these differences were statistically significant (p<0.05). Lack/inadequate knowledge, unavailability of reporting forms and negative attitudes were barriers identified; and addressing them resulted in significant improvement in this setting. Scaling up these interventions to other hospitals can better the situation of under-reporting of ADRs in Nigeria.
Keywords
Knowledge,Attitudes, Practices,ADRmonitoring, Health workers, Nigeria
Introduction
Antiretroviral therapy (ART) is usually a triple combination regimen of two nucleoside reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibi- tors (NNRTIs) or protease inhibitors in Nigeria. ART has been shown to maximally and durably suppress viral replication, and restore and/or preserve immune function as reflected by CD4 cell measures, thereby sig- nificantly reducing morbidity and mortality associated with HIV infec- tion, improving quality of life of HIV-infected patients and reduce risk of HIV transmission [1, 2]. However, ART is also associated with toxicities from the individual antiretroviral drugs in the regimen like any other drugs. Anaemia, hepatotoxicity, vomiting, peripheral neuropathy, diar- rhoea, dizziness, paraesthesia, bad dreams and skin rash were common ADRs observed in patients on ART [3-6]. Other toxicities include lipod- ystrophy, lactic acidosis, and hyperlipidemia amongst others. These drug- induced toxicities not only have the potential to diminish quality of life of the patients but can adversely affect treatment adherence by patients’ re- fusal to take any ART for fear of toxicity [7-9]. Poor adherence to ART is associated with development of drug-resistant human immunodeficiency virus, increased disease progression and virologic failure; consequently, morbidity and mortality as well as the burden on health care increases [10-12]. WHO defined pharmacovigilance as ‘‘science and activities relat- ing to the detection, assessment, understanding and prevention of adverse effects or any other drug related problems’’. The ultimate goal of pharma- covigilance is to ensure safe and rational use of medicines, and prevent avoidable negative consequences of pharmacotherapy in patients [13]. Pharmacovigilance programs have led to regulatory decision to withdraw of several approved and licensed drugs from the market because of drug- induced toxicities [14, 15]. However, under-reporting of adverse drug reactions (ADRs) is one of the major problems associated with pharma- covigilance programs. Recent studies provided evidence of significant and widespread under-reporting of ADRs to spontaneous reporting systems including serious or severe ADRs [16]. It is likely that less than 10% of serious reactions are reported [14, 17]. In many countries including Ni- geria, the reporting rates are likely to be much lower [18]. Studies con- ducted in different settings including Nigeria reported poor knowledge, attitude and practices to ADR monitoring and reporting among health- care professionals [18-28]. High patient load, indifference, unavailability and/or inaccessibility of the ADR reporting form, lack of incentives for the reporters, not knowing what to report or even who should report and fear of allegation for reporting errors were factors that may responsible for the low reporting rates. Sometimes healthcare professionals fear that the acknowledgement of adverse reactions may reflect negatively on their competence or put them at risk of litigation. Some are reluctant to report adverse reactions because of doubts regarding the causal role of the drug. The commonest factors that militate against ADR reporting were lack of knowledge, unavailability of reporting forms, ignorance of the reporting procedure and indifference to ADRs reporting [18, 24, 25]. Majority of health workers were willing to practice pharmacovigilance if they are trained [25, 26].
It is vital that the safety of all medicines is monitored throughout its clinical use. Clarity of criteria for reporting, simple procedures and good motivational practice may be influential in addressing the problems in some if not all settings [29]. Improving the knowledge, attitudes and practices of healthcare professionals regarding ADR monitoring and reporting can better the situation of under-reporting of ADRs. This study evaluated the change in knowledge, attitudes and practices of healthcare professionals about ADR monitoring and reporting after six months of capacity building interventions in a Nigerian tertiary hospital.
Methods
Research Design
This was a longitudinal study that aimed to improve the knowledge, attitudes and practices of healthcare professionals about monitoring and reporting of ADR related to antiretroviral therapy (ART) in a public health facility in Nigeria. The healthcare professionals included medical doctors, pharmacists, nurses, laboratory scientists, medical record officers and social welfare officers participating in the ART program in the study site. There was a baseline assessment of the knowledge, attitudes and practices of healthcare professionals in the ART programs about monitoring and reporting of ADR related to ART using a semi-structured and study-specific instrument. The post-intervention assessment of the knowledge, attitudes and practices of same healthcare professionals about monitoring and reporting of ADRs was done after 6 months.
Setting
The study was conducted in Federal Medical Center (FMC) Owo, Ondo State, Nigeria. This is a 300 bed-capacity tertiary health facility and serves both insured and uninsured members of the general public within and outside the State. HIV comprehensive care services including ART are provided at no cost to the patients with funding support from PEPFAR through USAID. The healthcare professionals involved in the ART program were trained on HIV Comprehensive Care Services based on the Nigerian National HIV/AIDS treatment guidelines at commencement. The training package included topics as adverse effects of antiretroviral drugs without emphasis on ADR monitoring and reporting.
Intervention
The interventions included a five–day group training of the healthcare professionals (doctors, pharmacists, laboratory scientists, nurses, medical record officers and social welfare officers) on clinical pharmacovigilance for antiretroviral drugs using a standardized training manual available at: http://www.nafdac.gov.ng/index.php?option=comdocman &task=catview&gid=53&Itemid =57. The training was facilitated by the researchers and three trained assistants. A multidisciplinary Hospital- based ART Pharmacovigilance Committee (HAPC) that included medical doctors, pharmacists, nurses, medical record officer, laboratory scientists and social welfare officers was constituted and inaugurated by hospital management. HAPC was mandated to coordinate pharmacovigilance of antiretroviral drugs in the health facility. HAPC holds monthly feedback and safety data review meetings; and follow up patients with individual case safety reports (ICSRs) and submit to ICSRs to the National Pharmacovigilance Center. Standard Operating Procedure (SOP) for detection, evaluation and reporting ADRs related to ART that included the ADR screening form and a flowchart for ADR monitoring and reporting was disseminated and adopted by the health facility. The national ADR reporting form (the yellow form) was also provided for reporting of suspected ADRs (ICSRs) to the National Pharmacovigilance center at NAFDAC. The copies of ADR screening and reporting tools were deployed to all service delivery points through the hospital medical record system. The healthcare workers were also trained on the use of the job aids and tools. No monetary incentive was provided to the health workers involved in the study. After interventions, there was a monthly follow up monitoring of hospital pharmacovigilance program and provision of technical assistance as appropriate by the researcher.
Selection Criteria
All healthcare professionals (medical doctors, pharmacists, nurses, laboratory scientists, medical record officers and social welfare officers) participating in the ART program in the study site and consented to participate were eligible to be included in the study. The healthcare professionals who are involved in the ART program but unwilling to participate in the study, those who are not involved in the ART program or on leave or absence from duty during the study period were excluded. All other workers who are not medical doctors, pharmacists, nurses, laboratory scientists, medical record officers and social welfare officers in the study site were excluded.
Study Population, Sample and Sampling Methods
The study site was selected using purposive sampling technique. The population for the study included all 36 healthcare professionals (16 medical doctors, 5 pharmacists, 7 nurses, 3 laboratory scientists, 3 medical record officers and 2 social welfare officers) participating in the ART program. All the healthcare professionals participating in the ART program were purposively selected for the study.
Validity and Reliability of Instrument
The study-specific knowledge, attitude and practices (KAP) questionnaire was circulated to the technical experts and a biostatistician. It was discussed objectively and modified based on the feedbacks for content validity. In addition, it was pre-tested which also provided opportunity for the modification and validation of the study instrument. The site and participants involved in the pre-testing of the instrument were not included in the main study to avoid bias. The characteristics of the healthcare professionals used in the pre-testing were similar to the selected study participants.
Ethical Consideration
The ethical approval for this study was obtained from National Health Research Ethics Committee (NHREC), Abuja, Nigeria. Informed consent of the participants was also obtained. Confidentiality was assured by excluding participants’ identifier during analysis.
Data Collection
The instruments included semi-structured and study-specific KAP questionnaire that employed mainly a Likert-type scale, pre– and post– training questionnaire and participants’ training evaluation form. The KAP questionnaire was administered to the study participants (medical doctors, pharmacists, nurses, laboratory scientists, medical record officers and social welfare officers) at baseline (month 0) and 6 months post-intervention. The pre– and post – training questionnaire was administered to the healthcare professionals before and after the training intervention. The participants’ training evaluation form was administered immediately after the capacity building for the evaluation of the training by the participants. The administration of the instruments was done by the researcher and three trained assistants.
Data Analysis
Predictive Analytics SoftWare (PASW) statistics-18 software was used for data analysis. The responses including those on 5-point Likert scales were analyzed using descriptive statistics on the sample characteristics and questionnaire items. Likert rating scale was anchored as follows: strongly agree = 5, agree = 4, neutral = 3, disagree = 2, and strongly disagree = 1 or always = 5, most of the time = 4, neutral = 3, rarely = 2, and never = 0; negatively worded items were reverse coded so that higher scores represent higher knowledge, attitudes and practices. Factor analysis was performed using principal components extraction. Missing values in the factor analysis was handled using listwise deletion. Factors selected had eigenvalues greater than 1. Items with factor loadings ≥0.40 were considered significant, and loadings ≥ 0.50 were considered “very significant” [30]. Mean item scores were computed for the individual attitude items. Paired samples t-test was used to compare the participants’ knowledge, attitude and practice before and after interventions. One sample t-test was used to compare participants’ attitudes within group. A midpoint of 3.6 was used for the 5- point scale which was determined by adding all the scores and computing the average. Mean attitude scores above the midpoint of 3.6 were regarded as positive attitude while scores below this mid-point were regarded as negative attitude. The reliability analysis was determined using Cronbach’s alpha. All reported P values were 2-sided and P<0.05 used to determine statistical significance.
Results
Thirty six (36) healthcare professionals participated in the 5-day capacity building intervention; 22 (61.1%) were males and 14 (38.9%) were females. These healthcare professionals included 16 (44.4%) medical doctors, 5 (13.9%) Pharmacists, 7 (19.4%) nurses, 3 (8.3%) laboratory scientists, 3 (8.3%) medical record officers and 2 (5.6%) were social welfare officers. The mean age of the participants was 36.6 (95%CI, 34.5–38.7) years.
Knowledge of the participants before and after the training intervention
Of the 36 participants who completed the pre-training knowledge evaluation, only 35 (97.2%) of them completed the post-training knowledge evaluation. The participants’ mean pre- test score was 53.6% (95%CI, 44.6–63.6) which increased after the training to a mean post-test score of 77.1% (95%CI, 72.8–81.4); and the mean percent knowledge change was 146.9% (95% CI, 60.5–233.3). The knowledge increase was statistically significant (p=0.000). None of the participants reported to have received training on pharmacovigilance previously, except the pharmacists who remarked that some pharmacists in the pharmacy department had received a training that included management of ADRs related to antiretroviral drugs as a topic in a broader Pharmaceutical Care in HIV training package.
Participants’ Evaluation of the Training workshop
On a 5-point scale, the participants’ overall rating of the training workshop was 4.5 (95%CI, 4.3–4.7); the topics covered in the training was rated 4.9 (95%CI, 4.8–5.0); organization of learning experience was 4.3 (95%CI, 4.1–4.5); length or space of schedule was 4.2 (95%CI, 4.0–4.4); presentations and explanation was 4.4 (95% CI, 4.2–4.6); opportunity to participate in discussions was 4.7 (95%CI, 4.5–4.9); accessibility and availability of trainers for clarifications was 4.6 (95%CI, 4.4–4.8); transport and accessibility to workshop venue was 4.2 (95%CI, 3.9–4.5); and meals provided was rated 3.9 (95%CI, 3.7–4.1). The participants’ ratings of the content and relevance of the topics covered at the training workshop were 4.6 (95%CI, 4.4–4.8) for Drug regulation and pharmacovigilance in Nigeria; 4.6 (95%CI, 4.4–4.8) for Overview of pharmacotherapeutics of HIV/AIDS; 4.7 (95%CI, 4.5–4.9) for Drug interactions and pharmacovigilance; 4.8 (95%CI, 4.7–4.9) for Pharmacovigilance: Medication error and Adherence; 4.7 (95%CI, 4.5–4.9) for Establishing pharmacovigilance systems in a clinical setting; 4.8 (95%CI, 4.6–5.0) for Diagnosis and management of common ADRs related to ART; 4.5 (95%CI, 4.3–4.7) for Laboratory monitoring and pharmacovigilance; 4.5 (95% CI, 4.3 – 4.7) for Reporting to the regulatory authorities; 4.5 (95%CI, 4.3–4.7) for Understanding signal and benefit-risk determinations; 4.6 (95%CI, 4.4–4.8) for Monitoring and evaluation of safety data; 4.6 (95%CI, 4.4–4.8) for Standard operating procedure for detecting, evaluating and reporting ADRs and 4.6 (95%CI, 4.4–4.8) for Effective communications in pharmacovigilance.
Attitudes and Practices of Health Workers about ADRs monitoring and reporting
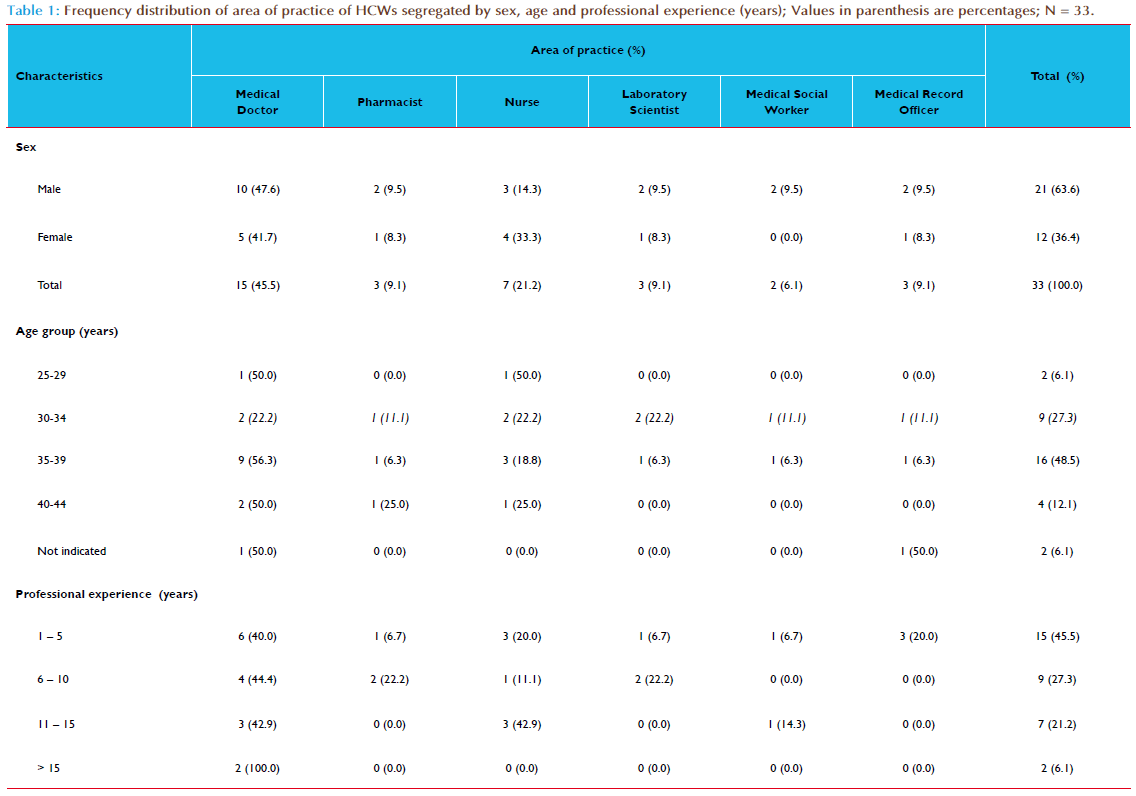
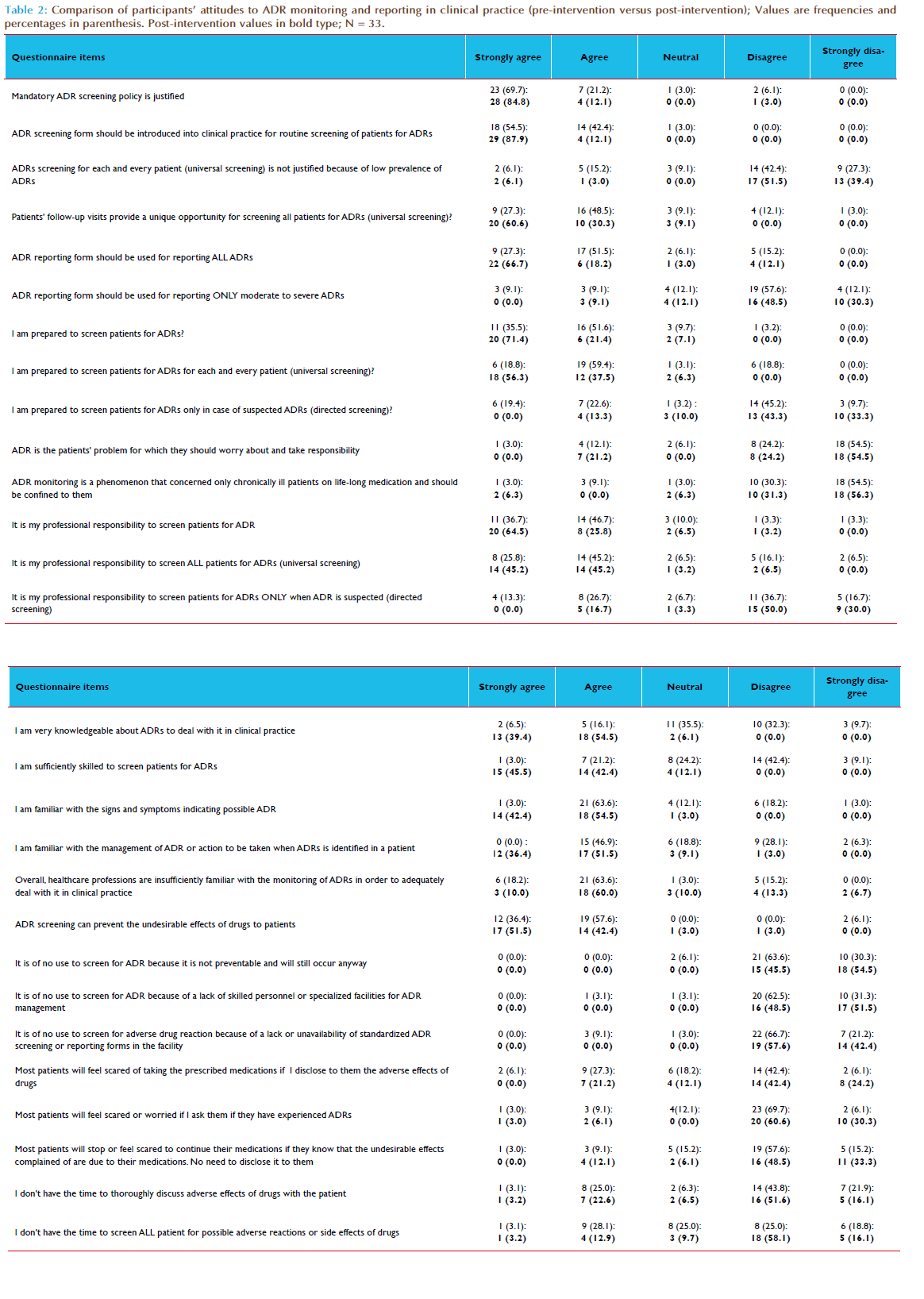
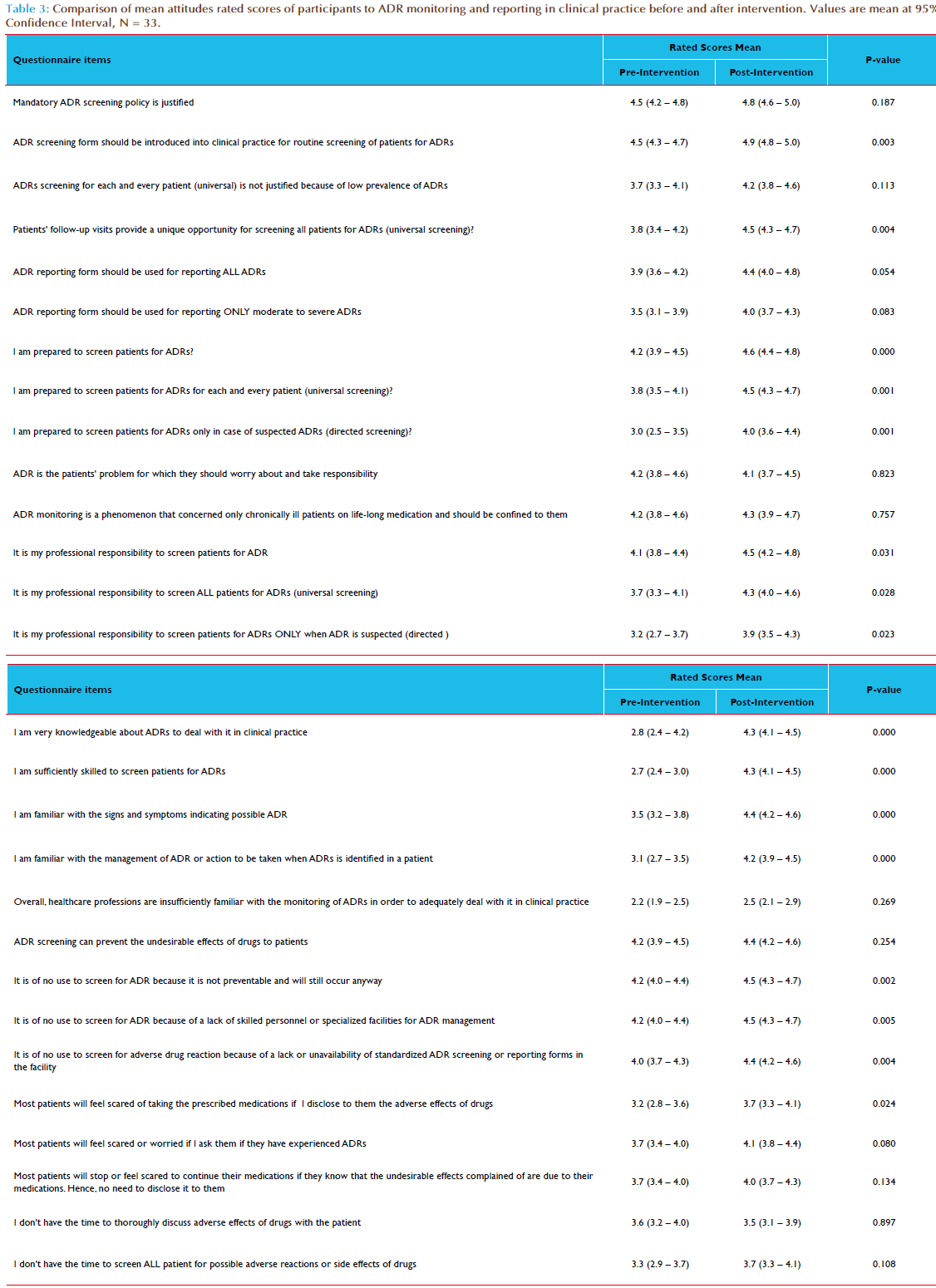
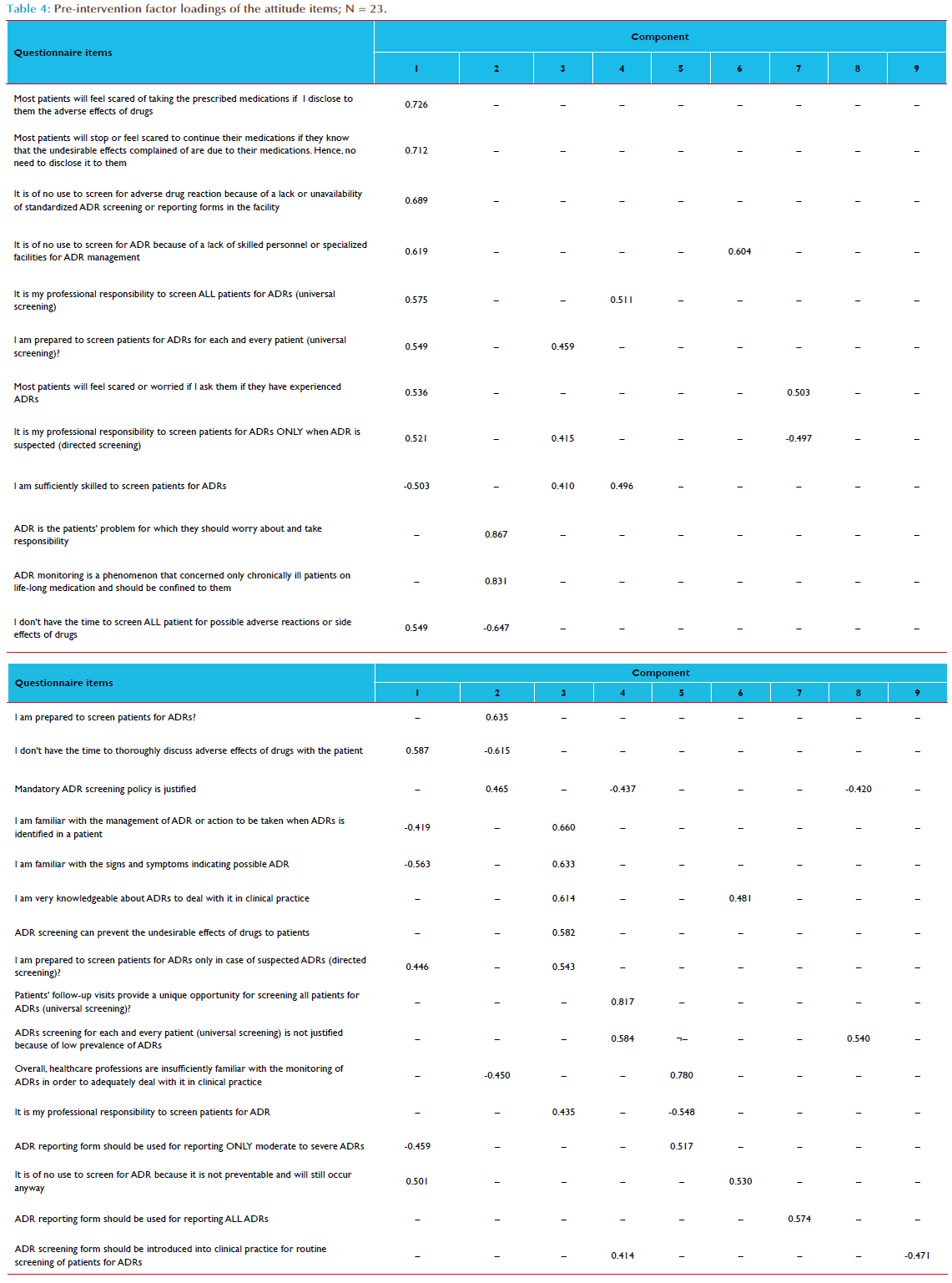
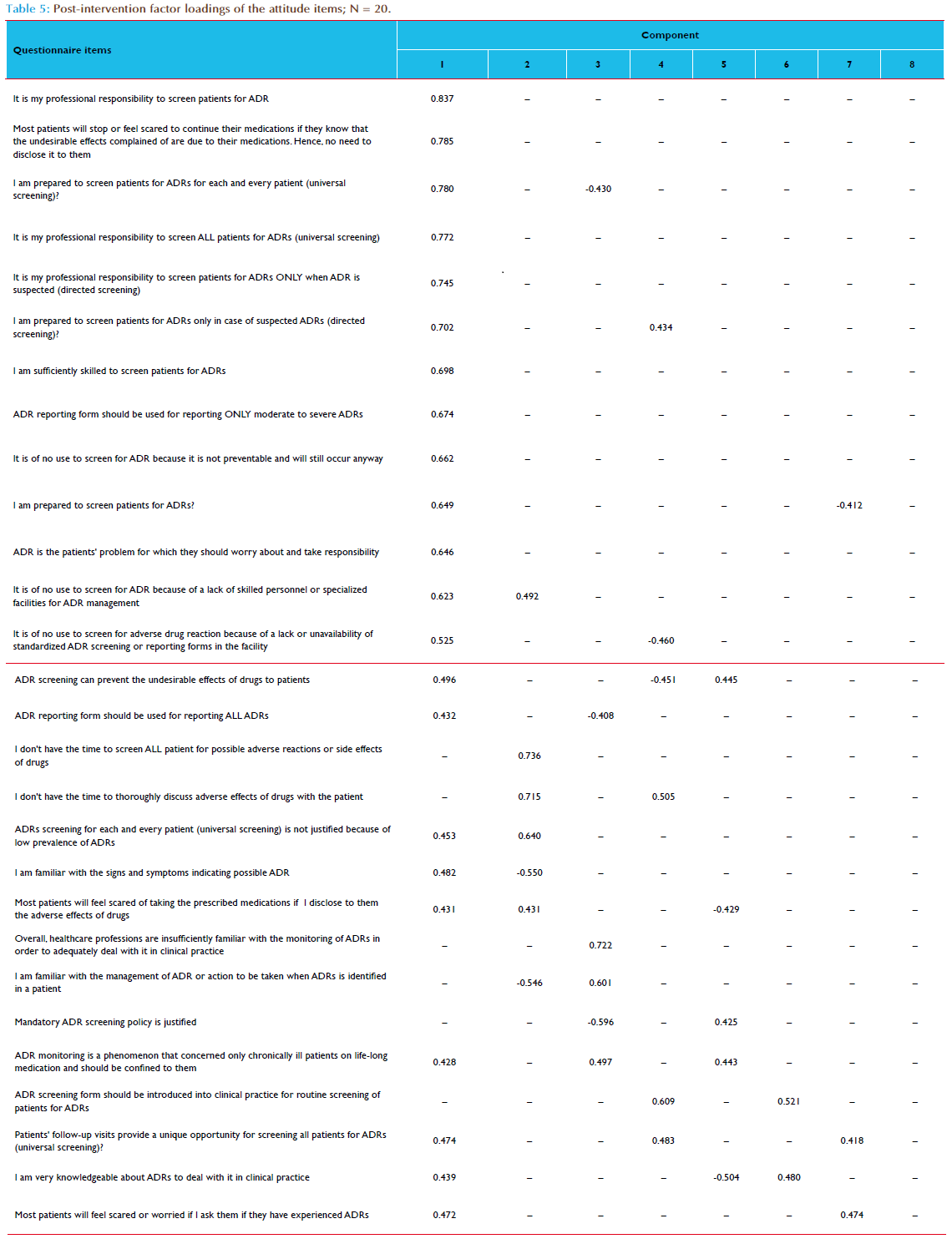
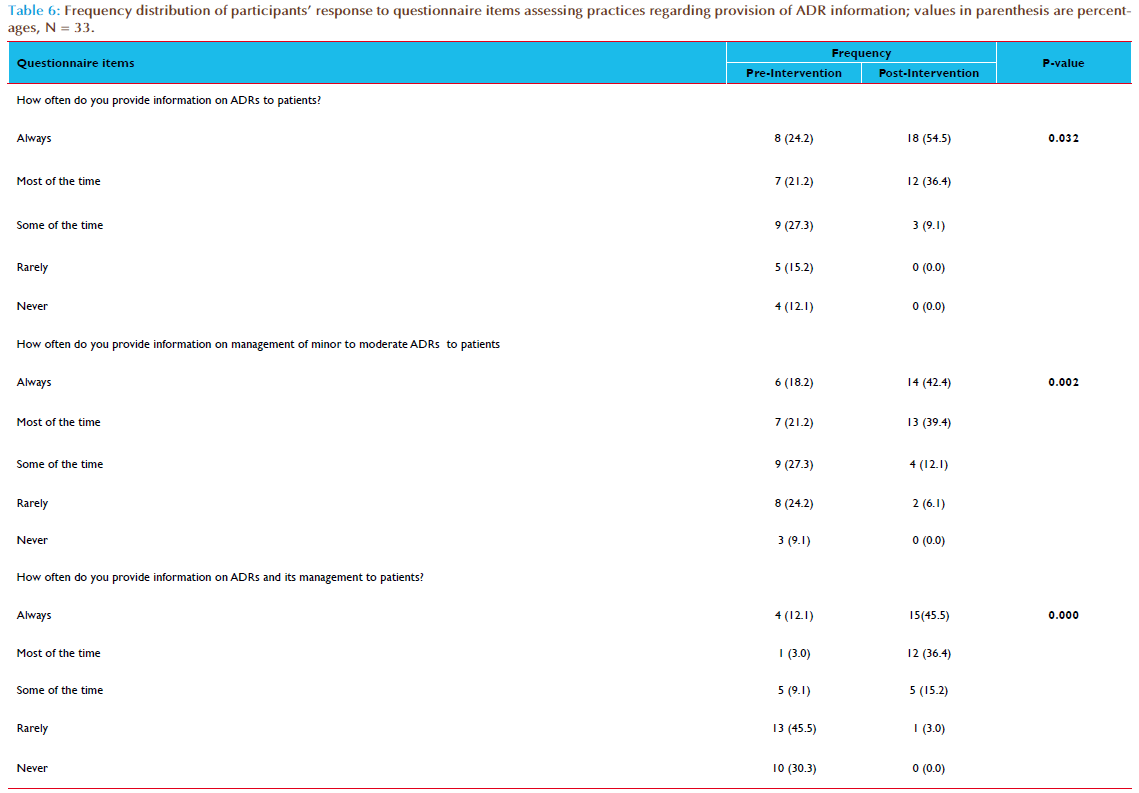
Of the 36 healthcare professionals who participated in the capacity building intervention, only 33 (91.7%) of them completed the KAP questionnaire at both pre-intervention (baseline) and 6 months postintervention. There were 21 (63.6%) males, 15 (45.5%) medical doctors and 25 (75.8%) were aged 30 – 39 years old (Table 1). The estimated mean number of patients’ contacts with participants per month was 521.4 (95%CI, 196.3–846.5). Table 2 compared the frequency distribution of participants’ attitudes towards ADR monitoring and reporting in clinical practice before and after interventions. The overall rated scores mean of the participants’ attitudes to ADR monitoring and reporting in clinical practice at pre-intervention was 3.6 (95%CI, 3.4–3.8; p=0.000); this increased to 4.2 (95%CI, 4.0–4.4; p=0.000) at post-intervention (Table 3). Overall, the difference in participants’ attitudes to ADR monitoring and reporting in clinical practice before and after interventions was statistically significant (p=0.000) – Table 3. The internal consistency of the 28-items attitude scale as measured by Cronbach’s alpha was 0.710; also the average measures of Intraclass Correlation Coefficient (ICC) was 0.710 (95%CI, 0.509–0.856; p=0.000). Following the listwise deletion of missing values, 23 and 20 cases were left for factor analysis at pre-intervention and post-intervention respectively. Using the criterion of an eigenvalue greater than 1.0, nine and eight factors were extracted which accounted for 85.6% and 87.0% of variance at pre-intervention and post-intervention respectively. All communalities at pre-intervention and post-intervention assessments were 0.70 or greater. At pre-intervention, the large first factor accounted for 21.1% of the variance. The second to ninth factors accounted for 14.6%, 11.8%, 10.5%, 7.5%, 6.2%, 5.6%, 4.6% and 3.7% of the variance, respectively. However, the scree plot indicated a break after the ninth factor (eigenvalue = 0.852). At post-intervention, the large first factor accounted for 30.9% of the variance. The second to eighth factors accounted for 14.6%, 10.6%, 9.1%, 7.2%, 5.8%, 5.2% and 3.6% of the variance, respectively. However, the scree plot indicated a break after the eight factor (eigenvalue = 0.910). All items had one factor loading of 0.40 or greater (Tables 4 and 5). At pre-intervention, only 10 (30.3%) of the participants reported the existence of a functioning hospital-based pharmacovigilance committee or any committee responsible for co-ordination of ADR monitoring and pharmacovigilance program. However, the participants reported that the committee was not multidisciplinary as it comprised mainly of medical doctors and pharmacists. The participants that reported the existence of the committee were 6 (40.0%) of the medical doctors, 3 (100.0%) of the pharmacists and 1(14.3%) of the nurses. On the other hand, 31 (93.9%) of the participants reported the existence of a multidisciplinary and functioning pharmacovigilance committee co-ordinating ART pharmacovigilance program in the hospital at post-intervention. Only two participants (a nurse and a social welfare of ficer) reported otherwise. The differences in the participants’ responses to the existence of a functioning hospital pharmacovigilance committee before and after intervention was statistically significant (p=0.001). Of the participants, 25 (75.8%) reported that ADR reporting form were not readily available in the hospital at pre-intervention assessment compared to 6 (18.2%) at post-intervention assessment. The differences in the participants’ response to the ready availability of ADR reporting form before and after intervention was statistically significant (p = 0.000). At pre-intervention, ADR reporting forms were not handled by the medical record officers who are responsible for the coordination of hospital medical records, opening of the patient’s hospital case folder and provision of all medical data collection and reporting tools. The medical record officers were not aware of the existence of ADR reporting forms. The ADR reporting form was mainly localized at the pharmacy and very scanty at the consulting clinics. At post-intervention, the participants reported a shift of the custody and provision of ADR reporting forms from pharmacy to medical record department for easy accessibility to all service delivery points. Of the participants, only 5 (15.2%) of them had reported ADR previously at pre-intervention assessment compared to 23 (69.7%) at post-intervention assessment. Of the participants who had reported ADR previously, only 2 (40.0%) kept record of all identified and reported ADRs at pre-intervention assessment, compared 22 (95.7%) reported at post intervention assessment. The participants who reported providing information regarding ADRs and its management routinely to patients increased from 4 (12.1%) at pre-intervention to 15 (45.5%) at post-intervention. This increase was statistically significant (p=0.000) - Table 6. Of the participants, 7 (21.2%) of the participants reported having standard operating procedure (SOP) for detecting, evaluating and reporting ADRs in the hospital at pre-intervention compared to 31 (93.9 %) at post-intervention. This increase was statistically significant (p = 0.012).
Discussion
The study evaluated the change in knowledge, attitudes and practices of healthcare professionals about ADR monitoring and reporting after six months of interventions. Majority of the participants were not trained on ADR monitoring and reporting previously. However, about onehalf of the participants had over 6 years of professional experience. The identified barriers to ADR monitoring and reporting in clinical practice at pre-intervention assessment were lack of knowledge, unavailability of reporting forms and ignorance of the reporting procedure.. These barriers were consistent with previous research findings [18, 24, 25]. Following the interventions, change in the participants’ knowledge of ADR monitoring and pharmacovigilance increased by over 100% after the training intervention which was statistically significant. This knowledge increase was consistent with the overall rating of the training workshop by majority of the participants as above average to excellent. The topics covered in the training, its content and relevance were rated as excellent by majority of the participants. In general, the participants reported negative attitudes to ADR monitoring and reporting in clinical practice at pre-intervention. This is consistent with previous research findings [18-28]. However, some of the participants had mean rated scores which denotes positive attitudes to ADR monitoring and reporting in some of the attitude items at pre-intervention. At post-intervention, almost all the participants had positive attitudes towards ADR monitoring and reporting in clinical practice. The difference in participants’ attitudes to ADR monitoring and reporting in clinical practice before and after interventions was statistically signifi- cant. However, the participants were inclined to having a mandatory ADR screening policy and the introduction of ADR screening form for active ADR surveillance in clinical practice at both pre- and postintervention. Similarly, the participants did not agree to the assertion that “healthcare professionals in general are insufficiently familiar with the monitoring of ADRs to adequately deal with it in clinical practice” at both pre- and post-intervention. This is consistent with the baseline pre-training knowledge assessment score of slightly over 50% recorded by the participants. Almost all the participants reported the existence of a functioning hospital-based pharmacovigilance committee or any committee responsible for co-ordination of ADR monitoring and pharmacovigilance program at post-intervention compared to less than onethird of the participants at pre-intervention. However, the participants who reported the existence of the committee at pre-intervention indicated that the membership was not multidisciplinary as it comprised mainly of medical doctors and pharmacists. This is contrary to a multidisciplinary and functioning hospital-based pharmacovigilance committee which held monthly ADR data review and feedback meetings at post-intervention. This difference was statistically significant and may have contributed to an improved coordination of ADR monitoring and reporting in the hospital at post-intervention. About three-quarter of the participants reported that ADR reporting form were not readily available in the hospital at pre-intervention assessment compared to less than one-fifth reported at the post-intervention assessment. This was a significant improvement as unavailability and lack of knowledge of the ADR reporting form were reported as causal to low ADR reporting rates in previous studies [24, 26-28]. The shift of the custody of ADR reporting form and its distribution to all service delivery points from pharmacy to medical record department which had no knowledge of this tool previously may have contributed to its ready availability at post-intervention assessment. At pre-intervention, less than one-sixth of the participants had reported ADR previously which is higher than what previous studies reported in Nigeria [26, 28]. However, this figure is lower than slightly over two-third of the participants who had reported ADR previously at post-intervention assessment and somewhat similar to what was reported in a developed country [33]. This increase was statistically significantly. Majority of the participants reported providing information regarding ADRs and its management routinely to patients at post-intervention compared to the baseline. Almost all the participants reported having SOP for detecting, evaluating and reporting ADRs in the hospital at post-intervention compared to about onefifth of participants at pre-intervention. The internal consistency of the attitude items was acceptable [31] and fairly superior to 0.70 indicating that the items are sufficiently correlated to constitute a scale [32]. All attitude items had one factor loading of 0.40 or greater which may indicate that the extracted factors represented the variables well [30]. The extracted factors at pre- and post-interventions reduced the complexity of the dataset with a 14.4% and 13.0% loss of information respectively. The sample sizes for each category of healthcare professionals were small and limited comparative analysis between groups. There may be response bias. Some participants may deliberately give false information to place themselves or the hospital in a good or bad position. This may overestimate or underestimate the measure of effects or the rated scores mean. There may also be selection bias when selecting the study site and participants. This may affect the generalization of the study findings. There may be recall bias by some participants when responding to the questions in the instrument. This has the potential to either overestimate or underestimate the effects been measured.
Conclusion
The study interventions resulted in significant improvement in ADR monitoring and reporting in clinical practice in this setting. Lack/inadequate knowledge, unavailability of reporting forms, and ignorance of the reporting procedure were barriers to ADR monitoring and reporting in clinical practice. Addressing these barriers led to significant improvement in knowledge, attitudes and practices of healthcare professionals about ADR monitoring and reporting in clinical practice. Healthcare workers were more willing to practice pharmacovigilance following the intervention. Scaling up these interventions to other hospitals can better the situation of under-reporting of ADRs in Nigeria.
Conflict of Interest Statement
We declare no conflict of interest.
References
- Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa AIDS 2004; 18: 887–95.
- Agu KA, Ochei UM, Oparah CA et al. Treatment Outcomes in Patients Receiving Combination Antiretroviral Therapy in Central Hospital Benin, Nigeria. Trop J Pharm Res. February 2010; 9(1): 1-10.
- Rajesh R, Vidyasagar S, Nandakumar K. Highly active antiretroviral therapy induced adverse drug reactions in Indian human immunodeficiency virus positive patients. Pharmacy Practice (Internet) 2011 Jan-Mar; 9(1): 48-55.
- Modayil RR, Harugeri A, Parthasarathi G, Ramesh M, Prasad R, Naik V, Giriyapura V. Adverse drug reactions to antiretroviral therapy (ART): an experience of spontaneous reporting and intensive monitoring from RT centre in India. Pharmacoepidemiol Drug Saf. 2010 Mar; 19(3): 247-55
- Singh H, Dulhani N, Tiwari P, Singh P, Sinha T. A prospective, observational cohort study to elicit adverse effects of antiretroviral agents in a remote resource-restricted tribal population of Chhattisgarh. Indian J Pharmacol. 2009; 41: 224–6)
- Olowookere SA., Fatiregun A.A., Akinyemi JO., Bamgboye AE., Osagbemi GK. Prevalence and determinants of nonadherence to highly active antiretroviral therapy among people living with HIV/AIDS in Ibadan, Nigeria. J Infect Developing Countries 2008; 2(5):369-372.
- Ammassari A, Murri R, Pezzotti P, et al. Self reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001; 28: 445–449.
- Fogarty L, Roter D, Larson S, et al. Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Educ Couns. 2002; 46: 93–108.
- Agu KA, Okojie O, Oqua D, et al. Medication Adherence and Risk factors for non-adherence among patients taking Highly Active Antiretroviral Therapy. West African Journal of Pharmacy 2011; 22 (1): 19 – 26.
- Este JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir Res. 2010; 85: 25–33.
- Protopopescu C, Raffi F, Roux P et al. Factors associated with non-adherence to long-term highly active antiretroviral therapy: A 10 year follow-up analysis with correction for the bias induced by missing data. J Antimicrob Chemother. 2009; 64: 599–606.
- Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008, 197 (Suppl 3): S272–278.
- Olsson S. The need for pharmacovigilance In: Gupta SK. Pharmacology and therapeutics in the new millennium. Narosa publishing house, New Delhi, 2001;502- 508
- Subish P, Mohamed Izham MI and Mishra P. Evaluation of the knowledge, attitude and practices on adverse drug reactions and pharmacovigilance among healthcare professionals in a Nepalese hospital: a preliminary study. The Internet Journal of Pharmacology. 2008. Volume 6 Number 1.
- World Health Organization, WHO. Safety of medicines: A guide to detecting and reporting adverse drug reactions. Geneva: 2002. WHO/EDM/QSM/2002.2
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006; 29(5): 385- 396.
- Lee A, Thomas SHL. Adverse drug reactions In: Walker R and Edward C. Eds.Clinical pharmacy and Therapeutics. 3rd Edition; Churchill Livingstone. 2003; 33 - 46.
- Okezie EO, Olufumilayo F. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008; 17(5): 517 - 522.
- Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: knowledge, attitude and practices of medical students and prescribers. Natl Med J India 2002; 15: 24 - 26.
- Li Q, Zhang SM, Chen HT, et al. FD. Study on the knowledge and attitude to adverse drug reactions reporting among healthcare professionals in Wuhan City. Zhonghua Liu Xing Bing Xue Za Zhi, 2004 Oct; 25(10): 894 – 897.
- Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom; Br J Clin Pharmacol. 1995 Mar; 39(3): 223- 226
- Khoza S, Madungwe I, Nyambayo P, et al. Adverse drug reactions reporting at a referral hospital in Zimbabwe. Cent Afr J Med. 2004 Nov-Dec; 50(11- 12): 104-7.
- Sevene E, Mariano A, Mehta U, et al. Spontaneous adverse drug reaction reporting in rural districts of Mozambique. Drug Saf. 2008; 31(10): 867-76.
- Fadare JO, Enwere OO, Afolabi AO, et al. Knowledge, Attitude and Practice of Adverse Drug Reaction Reporting among Healthcare Workers in a Tertiary Centre in Northern Nigeria. Trop J Pharm Res, June 2011; 10 (3): 237
- Oreagba IA., Ogunleye OJ, Olayemi SO. The knowledge, perceptions and practice of pharmacovigilance amongst community pharmacists in Lagos state, south west Nigeria. Pharmacoepidemiol Drug Saf. 2011; 20 (1): 30–35.
- Awodele O, Akinyede A, Adeyemi OA, et al. Pharmacovigilance amongst doctors in private hospitals in Lagos West Senatorial District, Nigeria Int J Risk Saf Med. 2011; 23(4): 217-226
- Ohaju-Obodo JO, Iribhogbe OI. Extent of pharmacovigilance among resident doctors in Edo and Lagos states of Nigeria. Pharmacoepidemiol Drug Saf. 2010 Feb;19(2): 191-195.
- Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clinical Pharmacology 2009; 9:14 doi:10.1186/1472-6904-9-14
- World Health Organization, WHO. Safety Monitoring of Medicinal Products.- Guidelines for setting up and running a Pharmacovigilance Centre. Uppsala Monitoring Centre- WHO Collaborating Centre for International Drug Monitoring, EQUUS, London, 2000; 10 – 11
- Hair JF, Anderson RE, Tatham RL. Multivariate Data Analysis, 2nd ed. New York, NY: Macmillan; 1987:149.
- Nunnally JC. Psychometric Theory. New York: McGraw-Hill, Inc., 1978.
- Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. Oxford: Oxford University Press, 1995.
- Hasford J, Goettler M, Munter KH, et al. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epi. 2002; 55(9): 945-950