In vitro Antibacterial Effect of three Intra Canal Medicaments with or without Electrophoresis on Enterocomlus faecalis at Different Dentin Depths
Received: 26-Jul-2023, Manuscript No. jbclinphar-23-108188; Editor assigned: 28-Jul-2023, Pre QC No. jbclinphar-23-108188 (PQ); Reviewed: 11-Aug-2023 QC No. jbclinphar-23-108188; Revised: 18-Aug-2023, Manuscript No. jbclinphar-23-108188 (R); Published: 25-Aug-2023
Citation: Azizlou E, Aminsobhani M. In vitro Antibacterial Effect of three Intra Canal Medicaments with or without Electrophoresis on Enterocomlus faecalis at Different Dentin Depths. J Basic Clin Pharma.2023,14(4):273-278.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Objective: Elimination of microorganisms from the root canal system plays a fundamental role in successful endodontic treatment. This study aimed to assess the antibacterial effect of three intra canal medicaments with/without electrophoresis on Enterococcus faecalis (E. faecalis) at different dentin depths.
Materials and methods: This study evaluated 81 sound extracted singlerooted, single-canal human teeth. After root canal preparation, the teeth were sterilized, and E. faecalis was inoculated into the root canals for 2 weeks. The teeth were then randomly divided into 9 groups (n=9) of Calcium Hydroxide (CH) without electrophoresis, CH with electrophoresis, Cupral without electrophoresis, Cupral with electrophoresis, silver nanoparticles (AgNPs) with CH without electrophoresis, AgNPs with CH and with electrophoresis, electrophoresis alone, negative control group, and positive control group. The medicaments were applied in the root canals and the teeth were incubated for 4 weeks. In electrophoresis groups, 15 mA electric current was applied for 6 min, and the teeth were then incubated. Then, dentin chips were collected from three different dentin depths by peeso reamers, and cultured on agar and thioglycolate culture media.
Clinical relevance: Increasing the penetration of intra canal medicaments into the dentin increases their antimicrobial effect.
Results: No significant difference was noted in antimicrobial activity of tested materials at different depths (P>0.05). A significant difference was noted in elimination of microbial biofilm between groups with and without electrophoresis at depths 2 and 3 (P<0.05).
Conclusion: Electrophoresis resulted in greater penetration of medicaments into dentinal tubules, and enhanced their antimicrobial efficacy in deeper areas of dentinal tubules.
Keywords
Calcium hydroxide, Electrophoresis, Enterococcus faecalis, Nanoparticle
Introduction
Proliferation of residual microorganisms in the complex root canal systems or the periradicular tissue is among the most important causes of endodontic treatment failures. Sum less of endodontic treatment greatly depends on elimination of microorganisms from the root canal system [1]. The most important factor among the causes of failure of endodontic treatments is the presence of Enterococcus faecalis (E. faecalis) in the root canal and is not removed by root canal therapy [2]. It can settle in the root canals and surrounding bone. This species may colonize the bone after extraction of an infected tooth and after placement of an implant in the healed site [3]. At present, chemomechanical debridement and application of medicaments in the root canal system in between treatment sessions are commonly practiced to achieve this goal [4]. Evidence shows that microorganisms lodged in the root canal porosities cannot be completely eliminated by instrumentation and use of irrigating solutions [5]. Chemical debridement of the root canal system is more important in root canals with difficult mechanical debridement such as open-apex teeth and those with thin dentinal walls. In such teeth, mechanical instrumentation further weakens the walls, necessitating a more effective chemical debridement [6]. Currently, CH is considered the first choice of intracanal medicament, has excellent antibacterial properties against common endodontic pathogens, but is less effective against E. faecalis [7]. Evidence shows that none of the currently available irrigating solutions and techniques can completely eliminate the microorganisms from the root canal system. In regenerative treatments, efficient cleaning of the root canal system is a challenge. On the other hand, techniques employed for root canal debridement should not damage the stem cells in the area [8]. Simultaneous use of antimicrobial agents can boost the antimicrobial activity and decrease the need for high dosage of antibacterial agents, which can subsequently decrease the side effects.
However, it should be noted that the efficacy of antimicrobial particles depends on their contact area. Recent advances in technology have enabled the possibility of size reduction of metal particles and their synthesis in micro- and nano-scales. By doing so, the surface area of metal particles increases, and their antimicrobial activity is enhanced [9]. Several factors affect the penetration depth of irrigating solutions into dentinal tubules, such as molecular size [10]. Use of nano-scale particles can increase the penetration depth of irrigating solutions. Use of metal nanoparticles for water disinfection has been previously reported. They can play an important role in water purification due to their high reactivity as the result of their high surface to volume ratio [11]. Copper nanoparticles have optimal antibacterial activity. The United States Environmental Protection Agency has confirmed the antibacterial activity of copper against potentially life-threatening microbial infections. Also, it has been reported that copper has the potential to interfere with the function of microorganisms in many ways, since several mechanisms decrease the ability of microorganisms to develop resistance against copper [12]. Silver Nanoparticles have strong antimicrobial activity against the bacteria, viruses, and fungi due to their small size and high surface area [13]. AgNPs are also used as an antifungal, anti-inflammatory and anti-viral agent [14-16]. Evidence shows that addition of copper and silver can increase the antimicrobial activity of CH [17,18]. Cupral is a combination of CH and copper (Humanchemie, Germany), which was introduced by Knappvost [18]. It has a stable balanced system and contains negatively charged copper hydroxide II nanoparticles and hydroxy coppurate anions, with high antimicrobial activity. It is effective against all aerobic and anaerobic microoganisms. Cupral can eliminate the resistance of microflora and has an antimicrobial activity 100 times stronger than that of CH [17]. Silver is also used to improve the properties of CH. Evidence shows that addition of copper and silver to CH can increase its antimicrobial activity [17,19]. Another way to increase penetration depth of irrigating solutions into dentinal tubules is to use electrophoresis. In endoodntics, electrophoresis is used to increase the efficacy of intracanal medicaments. Application of electric current can increase the penetration depth of CH and copper into dentin [19]. This study aimed to assess the effect of three antibacterial agents with/without electrophoresis on Enterococcus faecalis (E. faecalis) at different dentin depths.
Materials and Methods
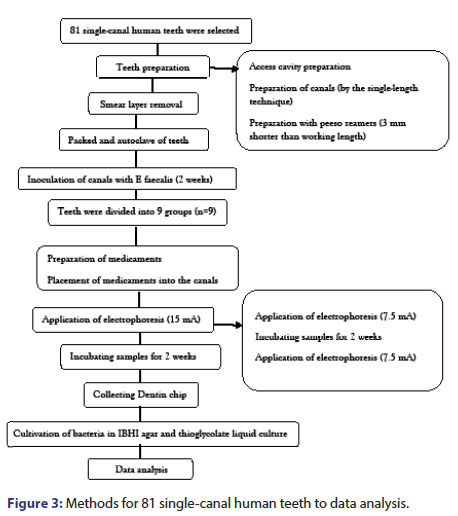
This experimental in vitro study evaluated 81 single-canal human teeth that had been extracted as part of periodontal or orthodontic treatment. The study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY.REC.1398.118). The medicaments evaluated in this study included CH (Golchadent, Tehran, Iran), Cupral (HUMANCHEMIE, Alfeld, Germany), and CH plus 5% AgNPs (nano-sized metal particles with 20 nm dimensions, US Research nanomaterials, Huston, USA). Preparation of teeth All teeth underwent radiography with digital sensor (RVG 5100, Care Stream Dental LLC, and Atlanta, GA, USA) from the buccolingual and mesiodistal directions (CS Imaging software). The teeth were radiographically examined. Also, they were inspected under a dental microscope (Carl Zeiss, Oberkochen, Germany) at X4 magnification for presence of cracks on the root surface.
Inclusion and exclusion criteria
Single-rooted single-canal teeth with straight canals, mature apices, no internal or external root resorption, no cracks on the root surface, and no caries on the root surface were selected. The teeth with oval-shaped canals and those with a length shorter than 20 mm were excluded and replaced. To eliminate the soft tissue from the root surface, the teeth were immersed in 5.25% sodium hypochlorite for 30 min, and stored in saline until the experiment. Next, for the purpose of standardization of root canals, the incisal edge of the teeth were reduced by a diamond fissure bur and high-speed handpiece such that all teeth had 15 mm length. Next, the access cavity was prepared by high-speed handpiece under air and water spray. To determine the working length, a#15 K-file was introduced into the canal until its tip was visible at the apex. Working length was determined to be 1 mm shorter than this length. All root canals were prepared by the same technique to minimize the confounding factors. All root canals were prepared by the singlelength technique using Endo-Mate DT rotary motor (NSK, Japan) and S1 to F3 ProTaper Gold rotary files (Dentsply Maillefer, Ballaigues, Switzerland) to the working length. Next, #1 and 2 (#70 and 90) peeso reamers were used to widen the canals to 3 mm shorter than working length. Accordingly, all root canals had the same diameter in 3-11 mm of the working length. During the instrumentation period, 1 ml of 2.5% sodium hypochlorite (Morvabon, Tehran, Iran) was used for irrigation after using each file. After completion of root canal preparation, 2 ml of 2.5% sodium hypochlorite was used for 10 minutes. Subsequently, canals were flushed with 10 ml of sterile normal saline. Then 2 ml of 17% ethylenediaminetetra-aceticacid (Morvabon, Tehran, Iran) was used for 1 minute to eliminate the smear layer. A final rinse with 10 ml of saline was then performed to eliminate the effect of disinfecting agents [20]. The teeth were then packed and autoclave-sterilized at 121°C for 30 min [21]. After sterilization, two teeth were randomly selected and incubated in brain heart infusion broth (Merck, Darmstadt, Germany) at 37°C for 48 h to ensure complete sterilization of the teeth. Next, inoculation of canals with E. faecalis was performed in all teeth, except for the negative control group.
The process of microbial inoculation of root canals was as follows, For E. faecalis biofilm formation, the methodology with slight modifications was followed [22]. In brief, the 24 h culture of E. faecalis in the stationary phase was diluted with 1% ratio (vol/vol) with tryptic soy broth containing 1% glucose, and 50 mg/L calcium chloride. This suspension contained 108 Colony Forming Units (CFUs) per milliliter, and 500 μL of this suspension was inoculated into a tube containing the teeth under sterile conditions. The suspension was refreshed every 48 h. According to the literature biofilm forms within 24 h [22-24]. The teeth in the tubes were incubated at 37°C for 14 days to allow biofilm formation and its penetration into dentinal tubules. Formation of biofilm was confirmed by random culture of some samples. According to the results of the Fuss study, using the One Way ANOVA Power Analysis option of the PASS II software, considering alpha equal to 0.05 and beta equal to 2 [19]. The standard deviation of the logarithm of the average colony number is 1.65 and the effect size is 0.46. The minimum sample size required in each of the 9 studied groups is 9 (total 81 teeth).
The teeth were then randomly divided into 9 groups (n=9):
Group I: CH without electrophoresis
Group II: CH plus copper (Cupral) without electrophoresis
Group III: CH plus 5% AgNPs without electrophoresis
Group IV: CH with electrophoresis
Group V: CH plus copper (Cupral) with electrophoresis
Group VI: CH plus 5% AgNPs with electrophoresis
Group VII: Electrophoresis alone
Group VIII: Positive control group to ensure presence of E. faecalis after culture
Group IX: Negative control group to ensure sterility of the teeth
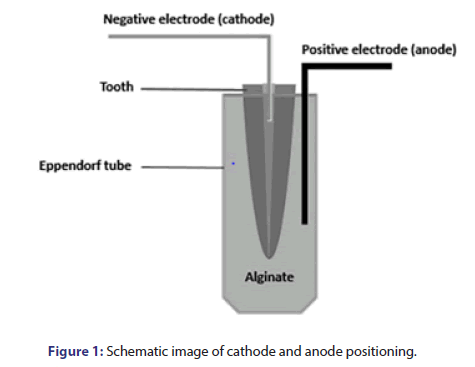
Preparation of medicaments was as follows: A total of 1000 mg of CH (Ca(OH)2); Golchadnet, Tehran, Iran] was mixed with 1 mL of sterile saline (Darou Pakhsh Pharmaceutical Mfg. Co, Tehran, Iran). To prepare CH paste plus 5% AgNPs, 950 mg CH was mixed with 50 mg AgNPs and 1 mL of sterile saline. Cupral paste was readily available. The medicaments were delivered into the canals by a #30 Lentulo spiral (Dentsply, Sirona, USA). For electrophoresis, the samples were placed in alginate (for electric current transfer) [25]. Depotphorese®-Gerät Original II (Humanchemie, Germany) was used to transfer electric current and perform electrophoresis. The positive electrode (anode) was placed outside the canal and in the alginate, while the negative electrode (cathode) was placed in the canal filled with medicament at 3-4 mm depth (Figure 1). The electric current transfer in electrophoresis groups was such that 7.5 mA was applied during 2.5 min. The teeth were then incubated for 2 weeks. After 2 weeks, 7.5 mA current was applied. In total, 15 mA was applied within 5 min. Next, the samples were incubated for 2 weeks. In groups without electrophoresis, only the medicaments were applied into the canals with #30 Lentulo spiral, and they were then incubated for 4 weeks. Finally, they underwent microbial analysis.
Microbial tests: After incubation of the samples, the 3 mm of apical third of the roots were cut by a diamond disc (thickness: 0.2 mm) (D+Z, Germany) and a micro motor (NSK, Japan). Also, the coronal parts of the roots were cut by a diamond disc to create an 8 mm cylinder (Figure 2). Next, the canals were rinsed with saline. To assess the efficacy of different techniques for inactivation of microbial biofilm and elimination of residual bacteria from the dentinal tubules, dentin chips were collected from the root canal walls by using sterile peeso reamers; #3 (#110) peeso reamer was used for depth 1, #4 (#130) was used for depth 2, and #5 (#150) was used for depth 3. Next, the dentin chips obtained at each phase were weighed, and 0.0005 mg of each was transferred into sterile 0.2 ml Eppendorf tubes containing sterile saline. They were then incubated at 37°C for 24 h to increase the number of viable bacteria; 100 μL of the obtained bacterial suspension was placed in brain heart infusion agar plates and also in thioglycolate liquid culture medium and were incubated at 37°C for 24 h. Next, the number of CFUs/mL was counted and proliferation of microorganisms was evaluated in thioglycolate liquid culture medium. The data were analyzed using PASS 11 software (Figure 3).
Results and Discussion
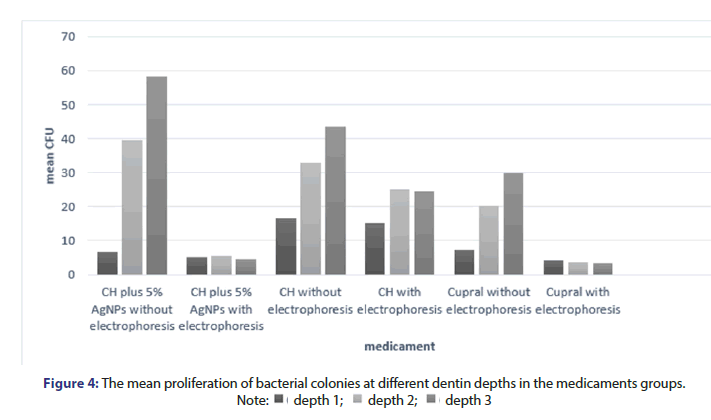
Table 1 shows the difference in microbial colony count in different antibacterial agents. As shown, in intergroup analysis, no significant difference was noted in reduction of colony count between the medicaments. Intra group analysis is shown in Figure 4. Figure 4 shows the mean proliferation of bacterial colonies at different dentin depths in the medicaments groups. As shown, no significant difference was noted between the effects of materials at depth 1. Also, no significant difference was noted in this respect between conduction and no conduction of electrophoresis at this depth. No significant difference was noted between the effects of materials at depths 2 and 3. However, a significant difference was noted between conduction and no conduction of electrophoresis at depths 2 and 3. In electrophoresis group alone, the proliferation of bacterial colonies at all depths was similar to that in the positive control group. The use of electrophoresis leads to a significant penetration of medicaments in the dentinal tubules and also increases the antibacterial effect against E. faecalis biofilm. Also, while Cupral had more antimicrobial effect, there was no significant difference (Table 1 and Figure 4).
| Study groups | Mean difference | Std error | P-value | |
|---|---|---|---|---|
| AgNPs+CH | CH | -10 | 5.46816 | 0.171 |
| Cupral | 0.2222 | 5.46816 | 0.999 | |
| Cupral | AgNPs+CH | -0.2222 | 5.46816 | 0.999 |
| CH | -10.2222 | 5.46816 | 0.159 | |
| CH | AgNPs+CH | 10 | 5.46816 | 0.171 |
| Cupral | 10.2222 | 5.46816 | 0.159 | |
Table 1: Difference in microbial colony count in different groups.
This study aimed to assess the antimicrobial efficacy of CH, AgNPs+CH and Cupral at different dentin depths with/without electrophoresis. CH is an intracanal medicament with antibacterial properties, which is extensively used in endodontic treatment [26,27]. The application of CH, has been questioned by some studies. Some evidence exists regarding the inactivation of antibacterial activity of CH by dentin [28]. Also, it has been reported that E. faecalis is resistant to CH [26]. Treatment with Cupral has gained the spotlight due to its strong antimicrobial activity [23]. Not only the proliferative bacteria, but also the bacterial spores and viruses are affected by Cupral due to its broad- spectrum antimicrobial efficacy [18]. On the other hand, the role of copper in angiogenesis has been previously documented [24]. This property of copper can help in regenerative treatments; however, further studies are warranted in this respect. AgNPs were also evaluated in this study since many studies have documented their optimal antibacterial activity against Gram-positive and Gram-negative bacteria [29-31]. E. faecalis is a Gram-positive obligate anaerobe and among the most resistant microorganisms causing endodontic infections [32]. It can form biofilm and penetrate into dentinal tubules [33]. Thus, it was used in the form of biofilm in this study. Biofilm forms within 24 h. in this study the teeth were incubated at 37°C for 14 days to allow biofilm formation and its penetration into dentinal tubules. This time period for biofilm formation was selected according to an ex vivo study that showed E. faecalis formation on root dentin [17]. Single-rooted teeth were evaluated in this study and their apical third was cut by a disc due to the presence of high anatomical variations and accessory canals in this region [34]. The root canals were prepared with ProTaper Gold rotary system up to F3 in all samples, and then #1 and #2 peeso reamers were used to standardize the canal volume. Non-invasiveness and low cost are among the advantages of electrophoresis [35]. In the clinical setting, electrophoresis can be used to deliver the medicaments into dentinal tubules. However, the patient’s tolerance threshold should be taken into account. In this study, we used 2.5 mA electric current, which was in accord with the manufacturer’s instructions, and within the tolerable range by patients [18]. Also, agar and thioglycolate culture media were used for evaluation of antimicrobial effects. The reason for E. faecalis biofilm culture in thioglycolate culture medium is because of the fact that E. faecalis is viable but not cultivable. In other words, E. faecalis cannot proliferate in the culture medium but preserves its viability and pathogenicity and starts to proliferate whenever the optimal conditions are provided [36]. The thioglycolate culture medium is a yellow, liquid culture medium that contains resazurin (an oxidation-reduction indicator). By addition of nutrients such as casein, yeast extract, vitamins, and dextrose to this medium, proliferation of pathogenic bacteria can be enhanced. Thus, inactive bacteria (due to inappropriate environmental conditions) are activated and proliferate as such [37]. The thioglycolate culture medium, which is an enriched medium was used in this study to ensure the accuracy of results. The results indicated that all three tested materials had optimal antibacterial effects with no significant difference between them. Fuss, et al. reported that Cupral had significant antimicrobial effects in their study, which was different from our results [18,19]. This difference may be due to the fact that Fuss, et al. used bovine teeth, which have differences with human teeth in terms of shape and anatomy. Dentinal tubules in bovine teeth are larger than those in human teeth [38,39]. Yousefshahi, et al. reported that both Cupral and AgNPs had higher antimicrobial activity than CH, which was different from our results [39,40]. This difference may be due to the fact that they directly applied the medicaments in the culture medium while we used extracted human teeth to assess the antimicrobial activity of medicaments. It should be noted that copper may have toxic effects on the periradicular tissues. Thus, in vitro and animal studies are required to further scrutinize the effects of copper on periradicular tissues [41]. Although AgNPs have significant antimicrobial effects, they can cause tooth discoloration [42]. Moreover, allergy to silver has been reported, which should be taken into account [43]. Electrophoresis is a type of electrotherapy during which, a substance is used for its local and systemic therapeutic effects at tissue depth [44]. Its mechanism of action is based on the fact that in a certain electric field, positively charged medicament ions (cations) are repulsed by the positive electrode (anode), and attracted by the cathode. The negatively charged ions (anions) that are repulsed by the negative electrode (cathode) are attracted by the anode. The molecules suitable for use with electrophoresis are small and hydrophilic [45]. Electrophoresis allows greater access of polar therapeutic molecules to deep skin areas by 10-2000 times compared with the conventional application of a medication [46]. In our study, groups that underwent electrophoresis showed higher antibacterial efficacy than the groups without electrophoresis. Also, at depths 2 and 3 of electrophoresis groups, bacterial biofilm was considerably lower than that in groups without electrophoresis, which can be attributed to the mechanism of action of electrophoresis [44-48].
Conclusion
The penetration depth of different medicaments and irrigating solutions into dentin is variable in root canal treatment. For example, a previous study reported the penetration depth of sodium hypochlorite to be 81- 376 μm. The penetration depth of CH in different parts of the root ranged from 28-125 μm in another study. Also, a previous study reported the penetration depth of CHX to be 44-138 μm. In our study, considering the preparation with peeso reamers, the penetration of materials to 300 μm depth with electrophoresis was expected. Future studies are required to compare the penetration depth of different medicaments. Limitation of this study were the high cost of the materials used and the tests to check the effectiveness of the drugs investigated in the research and Difficulty in coordinating with the microbiology laboratory and research center. Electrophoresis enhanced the penetration of tested medicaments into dentinal tubules and increased their antimicrobial efficacy deep in dentinal tubules. Although Cupral showed higher antimicrobial activity against E. faecalis biofilm, this difference was not significant. However, other clinical studies are needed in the future to confirm the results of in vitro studies.
Author Contributions
Mohsen Aminsobhani-planning and supervised the work, Data collection, microbiology analysis; Elaheh Azizlou-Data collection and processing, aided in interpreting the results and worked on the manuscript, Corresponding Author.
Availability of Data and Materials
Data generated or analyzed during this study are available upon request from the corresponding author.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We would like to express our sincere gratitude to our advisors, Dr. Kazem Ashofteh, for their invaluable guidance and support throughout the research process.
Ethics Approval and Consent to Participate
IR.TUMS.DENTISTRY.REC.1398.118.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997;23(12):725-777.
[Crossref] [Google Scholar] [PubMed]
- Alghamdi F, Shakir M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: A systematic review. Cureus. 2020;12(3).
- Flanagan D. Enterococcus faecalis and dental implants. J Oral Implantol. 2017;43(1):8-11.
[Crossref] [Google Scholar] [PubMed]
- Lim Z, Cheng JL, Lim TW, et al. Light activated disinfection: An alternative endodontic disinfection strategy. Aust Dent J. 2009;54(2):108-114.
[Crossref] [Google Scholar] [PubMed]
- Siqueira Jr JF. Aetiology of root canal treatment failure: Why well‐treated teeth can fail. Int Endod J 2001;34(1):1-0.
[Crossref] [Google Scholar] [PubMed]
- Diogenes AR, Ruparel NB, Teixeira FB, et al. Translational science in disinfection for regenerative endodontics. J Endod. 2014;40(4):S52-S57.
[Crossref] [Google Scholar] [PubMed]
- Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: A literature review-Part I. In vitro studies. Restor Dent Endod. 2014;39(4):241-252.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Song M, Kim E, et al. Pulp-dentin regeneration: Current state and future prospects. J Dent Res. 2015;94(11):1544-1551.
[Crossref] [Google Scholar] [PubMed]
- Holister P, Weener JW, Roman C, et al. Nanoparticles. Technology white papers. 2003;3:1-1.
- Giardino L, Ambu E, Becce C, et al. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J End. 2006;32(11):1091-1093.
[Crossref] [Google Scholar] [PubMed]
- Ichinose N, Ozaki Y, Kashu S. Superfine particle technology: Springer Science & Business Media; 2012.
[Crossref] [Google Scholar] [PubMed]
- Raffi M, Mehrwan S, Bhatti TM, et al. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol. 2010;60(1):75-80.
[Crossref] [Google Scholar] [PubMed]
- Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20(5):8856-8874.
[Crossref] [Google Scholar] [PubMed]
- Medda S, Hajra A, Dey U, et al. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci. 2015;5(7):875-880.
[Crossref] [Google Scholar] [PubMed]
- Hebeish A, El-Rafie M, El-Sheikh M, et al. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol. 2014;65:509-515.
[Crossref] [Google Scholar] [PubMed]
- Bekele AZ, Gokulan K, Williams KM, et al. Dose and size-dependent antiviral effects of silver nanoparticles on feline calicivirus, a human norovirus surrogate. Foodborne Pathog Dis. 2016;13(5):239-244.
[Crossref] [Google Scholar] [PubMed]
- Afkhami F, Pourhashemi SJ, Sadegh M, et al. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J Dent. 2015;43(12):1573-1579.
[Crossref] [Google Scholar] [PubMed]
- Fuss Z, Mizrahi A, Lin S, et al. A laboratory study of the effect of calcium hydroxide mixed with iodine or electrophoretically activated copper on bacterial viability in dentinal tubules. Int Endod J. 2002;35(6):522-526.
[Crossref] [Google Scholar] [PubMed]
- Ghasemi N, Torabi ZS. The effect of photodynamic therapy on the smear layer removal: A Scanning Electron Microscopic study. J Dentistry. 2021;22(3):162.
[Crossref] [Google Scholar] [PubMed]
- White RR, Hays GL. Failure of ethylene oxide to sterilize extracted human teeth. Dent. Mater. J. 1995;11(4):231-233.
- Haapasalo HK, Sirén EK, Waltimo TM, et al. Inactivation of local root canal medicaments by dentine: an in vitro study. Int. Endod. J. 2000 ;33(2):126-31.
- Sánchez-Sanhueza G, Alcántara-Dufeu R, Carrillo L, et al. Ex vivo effect of copper sulfate on Enterococcus faecalis in root canal. Int J Odontostomat. 2015;9(3):505-10.[ Crossref]
- Finney L, Vogt S, Fukai T, et al. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin Exp Pharmacol. 2009 ;36(1):88-94. .[ Crossref]
- Verma YK, Tripathi RP, Gangenahalli GU. Electrophoretic detection of alginate by Hematoxylin and Eosin fluorescence: Implications in cell encapsulation/tissue engineering. Reactive and functional polymers. 2016 ;102:130-6.
- Byström A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1(5):170-175.
- Chong BS, Ford TP. The role of intracanal medication in root canal treatment. Int Endod J. 1992;25(2):97-106.
- Portenier I, Haapasalo H, Rye A, et al. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34(3):184-188.
- Panáček A, Kvítek L, Prucek R, et al. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J Phys Chem. B. 2006;110(33):16248-16253. [Crossref]
- Baker C, Pradhan A, Pakstis L, et al. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5(2):244-249. [Crossref][Google Scholar][PubMed]
- Samiei M, Aghazadeh M, Lotfi M, et al. Antimicrobial efficacy of mineral trioxide aggregate with and without silver nanoparticles. Iran Endod J 2013;8(4):166.
- Valera MC, Silva KC, Maekawa LE, et al. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci. 2009;17:555-559.
[Crossref] [Google Scholar] [PubMed]
- Evans M, Davies JK, Sundqvist G, et al. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35(3):221-228.
[Crossref] [Google Scholar] [PubMed]
- Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol. 1984;58(5):589-599.
[Crossref] [Google Scholar] [PubMed]
- Karpiński TM. Selected medicines used in iontophoresis. Pharmaceutics. 2018;10(4):204.
[Crossref] [Google Scholar] [PubMed]
- Lleo MM, Bonato B, Tafi MC, et al. Resuscitation rate in different enterococcal species in the viable but non‐culturable state. J Appl Microbiol. 2001;91(6):1095-102.
[Crossref] [Google Scholar] [PubMed]
- Leijh PC, van Zwet TL, Ter Kuile MN, et al. Effect of thioglycolate on phagocytic and microbicidal activities of peritoneal macrophages. Infect Immun. 1984;46(2):448-452.
[Crossref] [Google Scholar] [PubMed]
- Schilke R, Lisson JA, Bauß O, et al. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol. 2000;45(5):355-361.
[Crossref] [Google Scholar] [PubMed]
- MS M. Comparação em MEV entre a dentina humana ea bovina. Pesqui. odontol. bras. 2001;15:80.
- Yousefshahi H, Aminsobhani M, Shokri M, et al. Anti-bacterial properties of calcium hydroxide in combination with silver, copper, zinc oxide or magnesium oxide. Eur J Transl Myol. 2018;28(3):7545.
[Crossref] [Google Scholar] [PubMed]
- Isani G, Falcioni ML, Barucca G, et al. Comparative toxicity of CuO nanoparticles and CuSO4 in rainbow trout. Ecotoxicol Environ Saf. 2013;97:40-46.
[Crossref] [Google Scholar] [PubMed]
- Moazami F, Sahebi S, Ahzan S. Tooth discoloration induced by imidazolium based silver nanoparticles as an intracanal irrigant. J Dent. 2018;19(4):280.
[Google Scholar] [PubMed]
- Raap U, Stiesch M, Kapp A. Contact allergy to dental materials. J Dtsch Dermatol Ges. 2012;10(6):391-396.
[Crossref] [Google Scholar] [PubMed]
- Ebisawa T, Nakajima A, Haida Het al. Evaluation of calcium alginate gel as electrode material for alternating current iontophoresis of lidocaine using excised rat skin. J Med Dent Sci. 2014;61(2):41-48.
[Crossref] [Google Scholar] [PubMed]
- Dhote V, Bhatnagar P, Mishra PK, et al. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci Pharm. 2012;80(1):1-28.
[Google Scholar] [PubMed]
- Kowalska M. Zastosowanie ketoprofenu w jonoforezie. Pediatria i Med. Rodz. 2011;7(2):124-128.
- Palazzi F, Blasi A, Mohammadi Z, et al. Penetration of sodium hypochlorite modified with surfactants into root canal dentin. Braz Dent J. 2016;27:208-2016.
[Crossref] [Google Scholar] [PubMed]
- Zand V, Mokhtari H, Hasani A, et al. Comparison of the penetration depth of conventional and nano-particle calcium hydroxide into dentinal tubules. Iran Endod J. 2017;12(3):366.
- Vadhana S, Latha J, Velmurugan N. Evaluation of penetration depth of 2% chlorhexidine digluconate into root dentinal tubules using confocal laser scanning microscope. Restor Dent Endod. 2015;40(2):149-154.


 depth 1;
depth 1;  depth 2;
depth 2;  depth 3
depth 3