Hepatoprotective effect of leaves of Peltophorum pterocarpum against paracetamol Induced acute liver damage in rats
- *Corresponding Author:
- Ramachandra Setty
College of Clinical Pharmacy, King Faisal University, Alahsa-31892, Saudi Arabia.
E-mail: rssiddamsetty@rediffmail.com
Date of Received: 06-01-2010
Date of Modified: 15-01-2010
Date of Accepted: 26-01-2010
Available Online: 15-02-2010
Abstract
The 70% ethanolic extract of Peltophorum pterocarpum leaves were in- vestigated for its hepatoprotective effect against paracetamol induced acute liver dam- age on albino wister rats. Paracetamol (2gm/kg, po) significantly elevated the serum levels of biochemical markers like SGPt, SGOt, ALP, bilirubin (total and direct), total cholesterol, triglycerides and depleted tissue GSH and increased the lipid peroxida- tion. upon administration of paracetamol (2mg/kg p.o.) to albino rats. this indicated that there the 70% ethanolic extract of leaves of Peltophorum pterocarpum at 100mg/kg and 200mg/kg doses significantly reduced the elevated levels of biochemical markers mentioned above. test extract treatment also increased the level of tissue GSH andsignificantly decreased tissue lipid preoxidation. the effect of 70% ethanolic extract (ELPP) was comparable with that of the standard silymarin 100mg/kg. these results suggest that 70% ELPP may have the potential therapeutic value in the treatment of paracetamol induced hepatic damage and some liver diseases. Hepatoprotective activity of the study plant may be attributed to the anti-oxidant principles in it.
Keywords
70% ELPP Hepatoprotectve GSH Lipid peroxidation Biochemical markers.
Introduction
Herbal medicines are being increasingly utilized to treat a wide variety of diseases, though the knowledge about their mode of action is relatively scanty. So there is a growing interest regarding the pharmacological evaluation of various plants used in traditional system of medicine. Many diseases (atherosclerosis, diabetis mellitus, asthma, nephritis, liver diseases) are due to the specific organ damage. The organ damage may be due to the excessive generation of free radicals [1]. Several drugs form natural source are known to scavenge off such free radicals and prevent the organ damage [2].
Liver is a major organ system involved in the metabolism of various drugs, xenobiotics and toxins. During the metabolism, excessive free radicals are generated and may cause liver damage. There are no drugs in the allopathic system of medicine to treat hepatic damage or to enhance the recovery of liver from damage. Therefore drugs from natural source are being adopted to treat hepatitis/liver diseases. In our search for effective and cheaper remedies for hepatic disorders, a wildly grown plant by name Peltophorum pterocarpum, which was claimed to possess hepatoprotective property, was found. Upon literature review it was found that the plant is traditionally used in the treatment of unhealthy skin, ringworm, constipation, insomnia, stomatis [7]. The leaves, bark and wood of plant contain tannins [5]. Tannins are natural anti-oxidants8. Phytochemical investigation showed the presence of flavonoids. The literature available on the plant is incomplete.
Paracetamol is widely used analgesic and antipyretic, produces acute liver damages at very larger dose. The hepatotoxicity of paracetamol has been attributed to the formation of toxic highly reactive metabolite n-acetyl parabenzoquineimine (NAPQI) [3]. Parcetamol also cause nephro toxicity [4].
The plant Peltophorum pterocarpum, belongs to family Leguminosae and is relatively virgin. The vernacular names of this plant: in Hindi as Pella Gulmohar [5,6], Bengali as Radha khrisnachura. Keeping the literature of the plant and the hypothesis that scavenging of free radicals and reactive oxygen species by natural anti-oxidants may protect the organ such as liver in the background the present study is planned to verify the hypothesis and establish utility of one more wildly grown plant in treating hepatic disorders.
Materials and Methods
Animals
Albino rats (Wistar) weighing 150–200g and albino mice weighing 20–25g of either sex were used in this study. They were procured from Sri Venkateshwara Enterprises, Bangalore. The animals were acclimatized for one week under standard husbandary conditions. They were fed with standard rat feed (Gold Mohur Lipton India Ltd.) and water ad libitum. Ethical clearance for experimental protocol and handling the animals was obtained from the Institutional animals ethical committee prior to the beginning of the project work.
Plant material
Peltophorum pterocarpum leaves were collected from the fields of Harapanahalli. The plant was identified and authenticated by Prof. K. Prabhu, Department of Pharmacognosy, S.C.S. College of Pharmacy, Harapanahalli. A herbarium specimen is deposited in our college museum. The leafs were shade dried at room temperature and pulverized.
Preparation of extract
The shade-dried powder was extracted directly with 70% ethanol (hydro-alcoholic extract), concentrated under reduced pressure. This extract was used for the study after subjecting it to preliminary qualitative phytochemical studies. The extracts were stored in a desicator until further use.
Preliminary phytochemical investigation
The preliminary phytochemical screening of 70% ethanolic extract of Peltophorum pterocarpum leaves was carried out for qualitative identification of type of phytoconstituents present [9,10].
Acute toxicity(LD50) study
The acute toxicity for 70% ethanolic extracts of Peltophorum pterocarpum leaves were determined on albino mice, maintained under standard conditions. The animals were fasted overnight prior to the experiment. Fixed dose method of OCED Guideline No. 420 given by CPCSEA was adopted for toxicity studies [11].
Animal treatment [12]
Albino rats of either sex weighing between 150 – 200 g were randomly assigned into 5 groups of 6 animals each. Group-I (Negative control) received 1ml/kg normal saline, Group-II(positive control), Group- III(standard silymarin 100mg/kg), Group-IV and V(test extract 100mg/kg & 200 mg/kg) were treated with respective treatments for 7 days, p.o. On 5th day 30 minutes after respective treatments, paracetamol 2g/kg orally was administered. After 48 hours of paracetamol challenge, blood samples were collected under mild ether anaesthesia; later the animals were sacrificed and liver tissues were collected. The blood samples were analysed for biochemical markers of hepatic injury and tissue samples were subjected for estimation of tissue glutathione (GSH) and lipid peroxidation (LPO).
The blood samples were drawn from all the animals by puncturing retro-orbital plexus on 7th day of the treatment. The blood samples were centrifuged immediately to get clear serum and subjected for estimation of various biochemical parametears namely SGPT (serum glutamic pyruvic transaminase), SGOT (serum glutamic oxaloacetic transaminase), ALP (alkaline phosphatase)., serum bilirubin (total and direct), total cholesterol, serum triglcerieds.
Invivo anti-oxidant
GSH estimation [19]
Tissue samples were homogenized in ice cold Trichloroacetic acid (1 gm tissue plus 10 ml 10% TCA) in a ultra turrax tissue homogenizer. Glutathione measurements were performed using a modifide Ellamn procedure (Aykae, et.all. 1985). Briefly, after centrifugation at 3000 rpm for 10 minutes, 0.5 ml of supernatant was added to 2 ml of 0.3 M disodium hydrogen phosphate solution. A 0.2 ml solution of dithiobisnitrobenzoate (0.4 mg/ml in 1% sodium citrate) was added and the absorbance at 412 nm was measured immediately after mixing. % increase in OD is directly proportional to the increase in the levels of Glutathione. Hence, % increase in OD is calculated.
Lipid peroxidation (LPO) estimation [20]
The degree of lipid peroxide formation was assayed by monitoring thiobarbituric reactive substance formation.
Combine 1.0 ml of biological sample (0.1–2.0 mg of membrane protein or 0.1–0.2 <mol of lipid phosphate) with 2.0 ml of TCA(Trichloroacetic acid) –TBA (Thiobarbituric acid)-HCl (Hydorchloric acid) and mix thoroughly. The solution was heated for 15 min in a boiling water bath. After cooling, the flocculent precipitate was removed by centrifugation at 1000 rpm for 10 min. The absorbance of the sample is determined at 535 nm against a blank that contains all the reagents minus the lipid.
Statistical analysis
Results were expressed as mean < SEM, (n=6). Statistical analysis was performed with one way analysis of variance (ANOVA) followed by Tukey-Kramer Multiple Comparisons Test by using Graph Pad Instat Software. P value less than 0.05 was considered to be statistically significant. *P<0.05, **>0.01 and ***<0.001, when compared with control and toxicant group as applicable
Result
Preliminary Phytochemical Screening
The phytochemical screening of extract showed the presence of tannins and flavonoids. It also showed the presence of alkaloids, saponins, carbohydrates.
Acute toxicity (LD50) studies
An attempt was made to determine LD50 of 70% ethanolic extract of leaves. Since no mortality was observed at 300 mg/kg. It was thought that 1000 mg/kg was the cut off dose. Therefore 1/10th and 1/5th dose (100 mg/kg, 200 mg/kg) were selected for all further in vivo studies.
Biochemical markers
Paracetamol challenge (as in negative control) elevated the levels of serum enzymes (SGPT, SGOT, ALP), serum bilirubin(total and direct)), total cholesterol, serum triglycerides. This indicates that paracetamol treatment caused liver damage. However 100 mg /kgand 200mg/kg extract significantly reduced the elevated levels of biochemical parameters significantly (p<0.05–p<0.001). Silymarin (100mg/ kg) also significantly decreased the levels of biochemical parameters. The results are compiled in table -1.
| Treatment | SGPT U/L | SGOT U/L | Total Bilirubin mg/dl | Direct Bilirubin mg/dl | Total Cho- lesterol mg/dl | ALP IU/L | Triglycerides mg/dl |
|---|---|---|---|---|---|---|---|
| Negative control (1ml vehicle p.o.) | 64.696 ± 5.088 | 67.16 ± 3.753 | 0.89 ± 0.077 | 0.213 ± 0.006 | 115.63 ± 5.406 | 131.68 ± 3.143 | 175.37 ± 5.670 |
| Paracetamol (positive control) (1ml vehicle p.o.+ 2 g/kg p.o.) | 263.170 ± 6.908 | 323.51 ± 8.251 | 4.217 ± 0.058 | 1.45 ± 0.056 | 185.36 ± 4.106 | 425.36 ± 8.282 | 210.56 ± 7.688 |
| Paracetamol +Silymarin (2 g/kg p.o. + 100 mg/kg, p.o.) | 78.567 ± 6.230*** | 93.47 ± 4.525*** | 1.092 ± 0.064*** | 0.296 ± 0.006*** | 118.67 ± 6.235*** | 169.93 ± 4.433*** | 179.17 ± 8.888** |
| Paracetamol +70% ethanolic extract (2 g/kg p.o.+ 100 mg/kg p.o.) | 121.613 ± 1.687*** | 151.36 ± 0.756*** | 1.27 ± 0.116*** | 0.323 ± 0.017*** | 146.49 ± 0.757*** | 241.49 ± 1.006*** | 192.77 ± 1.474 |
| Paracetamol +70% ethanolic extract (2 g/kg p.o. + 100 mg/kg p.o.) | 80.36 ± 1.022*** | 98.59 ± 0.928*** | 1.08 ± 0.430*** | 0.294 ± 0.011*** | 121.27 ± 1.332*** | 172.86 ± 0.969*** | 181.09 ± 0.913* |
Values are the mean ± S.E.M. of six rats/treatment. Significance *P<0.05, **P <0.01 and *** P<0.001 compared to Paracetamol treatment
Table 1: Effects of 70% ethanolic extract of Peltophorum pterocarpum leave s on bi ochemic al markers in parace tamol induced hepatotoxicity
GSH level
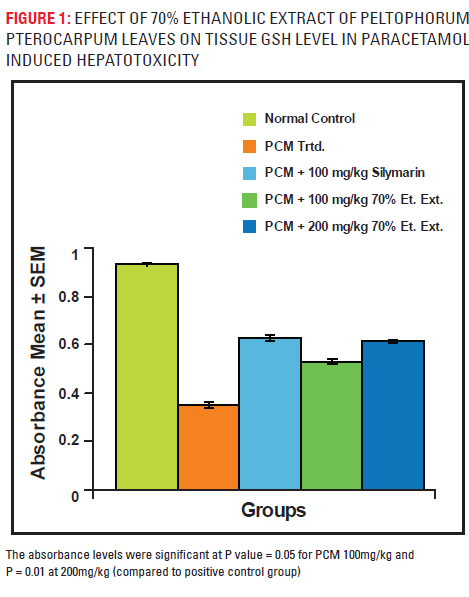
There was a marked depletion of GSH level in paracetamol treated group. 70% ELPP showed a dose dependent and significant increase in levels of tissue GSH. The results are compiled in Fig. – 1
Lipid peroxidation
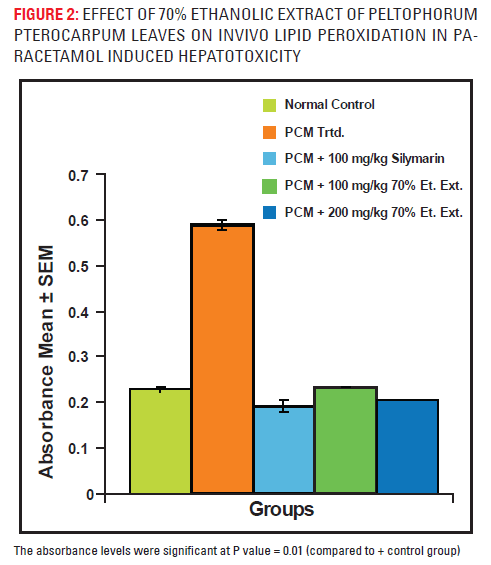
The effect of 70% ELPP on lipid peroxidation in paracetamol induced liver damage shown in fig. – 2. Treatment with 100 mg/kg and 200mg/kg extract significantly reduced the lipid peroxidation in a dose dependent manner.
Discussion
Paracetamol (N-acetyl-p-aminophenol) is a widely used analgesic and antipyretic drug and is safe when used in therapeutic doses. However, over dosage of paracetamol is known to be hepatotoxic and nephrotoxic in man and in experimental animals 3.
At lower doses, about 80% of ingested paracetamol is eliminated mainly as sulfate and glucoronide conjugates before oxidation and only 5% is oxidized by hepatic cytochrome P450 (CYP2E1) to a highly reactive and toxic electrophile i.e. N-acetyl-p-benzoquineimine (NAPQI). After over dosage of paracetamol the glucoronidation and sulfation routes become saturated and as a consequence, paracetamol is increasingly metabolized into NAPQI (4). Semiquinone radical, one-electron reduction metabolite of NAPQI mediate is the cause for the cytotoxic effects of NAPQI.These semiquinone radicals, in turn, can bind directly with cellular macromolecules to produce toxicity or alternatively, the radical can be reoxidized back to their original quinones by donating one electron to molecular oxygen under aerobic conditions. This donation of one electron then generates reduced oxygen radical species and hydroxyl radical. Both semiquinone and oxygen radical are known to be responsible for cytotoxic effects observed with quinones4.
Nevertheless at high doses of paracetamol NAPQI can alkylate and oxidize intracellular GSH and protein thiol group, which result in the liver GSH pool depletion and the reactive intermediate reacts with other nucleophilic centers of vital molecules in liver cells leading subsequently to hepatotoxicity. Besides, paracetamol is also shown to directly inhibit cellular proliferation, induce oxidative stress, resulting in lipid peroxidation, deplete ATP levels and alter Ca++ homeostasis; all of these changes are considered potentially fatal to the cell [21, 4].
In the present study the damage of liver due to paracetamol over dosage was confirmed by elevated levels of biochemical parameters like SGPT, SGOT, ALP, serum bilirubin (total and direct), total cholesterol, serum triglycerides. This is due to the fact that hepatic cells possess a variety of metabolic activities and contain a host of enzymes. SGPT, SGOT found in higher concentration in cytoplasm and SGPT particularly in mitochondria. In liver injury the transport function of hepatocytes is disturbed, resulting in the leakage of plasma membrane [22], thereby causing leakage of such enzymes leading to the increased serum levels of them. The elevated activities of SGPT, SGOT in paracetamol induced liver injury in serum indicative of cellular leakage and loss of functional integrity of cell membrane in liver.[24] SGPT is the best parameter than SGOT to justify the liver damage, since SGOT also present in kidney and cardiac muscle. Paracetamol are nephrotoxic, so they are damaging kidney cell and releasing SGOT to serum. In paracetamol induced liver damage the level of SGOT is more than SGPT [24,25].
Treatment with 70% ELPP decreased the elevated levels of SGPT, SGOT which may be a consequence of the stabilization of plasma membrane as well as repair of hepatic tissue damage caused by paracetamol. This may be supported by a view that the healing of hepatic parenchyma and regeneration of hepatocytes [26].
Serum ALP and bilirubin levels on the other hand are related to the function of hepatic cell. Increased in serum ALP level is due to increased synthesis in presence of billary pressure [27]. The extract shown dose dependent significant reduction in serum ALP and bilirubin (total and direct), indicating an improvement in the secratory mechanism.
Total cholesterol and serum triglycerides level also increased in paracetamol induced liver damage. Total cholesterol level increased may be due to the inhibition or destruction of triglycerides secretory mechanism by liver. 70% ELPP significantly reduced the level of total cholesterol and serum triglycerides.
Lipid peroxidation is a destructive process in liver injury due to paracetamol administration [27]. In this study the elevation in the level of TBARS in liver of rat were observed. The increase in TBARS level suggests enhanced lipid peroxidation during tissue damage and failure of anti-oxidant defense mechanism to prevent formation of excessive free radicals. 70% ELPP significantly inhibited the lipid peroxidation. It may possible that mechanism of inhibition of lipid peroxidation may be due to its anti-oxidant effect.
Glutathione is one of most abundant tripeptide anti-oxidant present in liver. It has been suggested this compound protect the thiol groups of protein from oxidation by free radicals. In present study paracetamol depleted the GSH level with an association of increased lipid peroxidation, which leads to tissue injury and liver damage. But 70% ELPP reversed the changes, increased the GSH level significantly. This infers the anti-oxidant characters of 70% ELPP.
70% ELPP possess tannins and flavonoids, which are natural anti-oxidant. They can scavenge off free radicals. So the increased level of GSH and decreased lipid peroxidation are because of presence of anti-oxidant principles. The anti-oxidant principles are involved in the organ protective activity.
In conclusion the results of study demonstrate that 70% ELPP possess hepatoprotective property. This property may be attributed to the anti-oxidant principles of the extract.
Further studies are required to identify, isolate, characterize and evaluate the active principal responsible for hepatoprotective activity of plant. The toxicological aspect of plant is not studied in this project, so toxicological assessment could be carried out.
Acknowledgements
We are thankful to the management of T.MA.E. Society for the constant encouragement and facilities provided be them.
References
- Maxwell AG, Masato Y and Yoko A. Free radical scavenging action of medical herbs from Ghana Thonningia sanginea on experimentally –induced liver injuries. General Pharmacology, 1999; 32: 661–667.
- Susanta KM, Goutam C, Gupta M and Majumder UK. Invitro antioxidant activity of Diospyros malabarica Kostel bark. Ind J Exp Bio. 2006; 4: 39–44.
- Dipak V, Parmar, Gazala Ahmed, Milind A, Khandkar A and Surendra SK. Mitochondrial AT - Pase: a target for paracetamol-induced hepatotoxocity. Eur. J. Pharmacol. 1995; 293: 225–229.
- Diadelis Remirez, Jan NM, Commandeur, Groot. Eds, Nico PE Vermeulen. Mechanism of protection of lobenzarit against paracetamol- induced toxicity in rat hepatocytes. Eur J Pharmacol. 1995; 293: 301–308.
- www.google.co.in Peltophorum pterocarpum (Dc) Hayne, Houerou
- www.google.co.in Peltophorum pterocarpum: yellow poinciana, Edward FG, Dennis GW, Nov 1993.
- www.botanical.com. Medicinal herbs of Chattisgarh, India having less known traditional uses 64, Peela Gulmohar. (Peltophorum pterocarpum, family-cesalpiniaceae) Oudhia Pankaj
- Ciddi V and Kaleab A. Antioxidants of plant origin. Ind. J. Nat. Prod. 2005; 21 (4): 3–17.
- Kokate CK. Practical Pharmacognosy 4th ed. New Delhi, Vallabha prakashan, 1999; 149–56.
- Khandelwal KR. Practical Pharmacognosy techniques and experiments, 2nd ed. Pune, Nirali Prakashan. 2000; 149–56
- Prema Veeraraghavan. Expert Consultant, CPCSEA, OCED Guideline No. 420.
- Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J. Ethnopharmacol, 2003; 89: 217–219
- Teitz NW, Expert Panel on enzyme of the IFCC. Clin Chem Acta, 1976; 70.
- Tietz NW, Rinker D and Show LM.IFCC method for alkaline phosphatase. J. Clin Chem Clin Biochem, l 1983; 21: 731–48.
- Burtis CA and Ashwood ER. Eds. Tietz. Textbook of Clin Chem. Philadelphia.W.B. Saunders Co. 1999; 3: 1829.
- Tietz NW, Text book of Clin Chem. Gambino, Michealson SR, Gambino MSR, et al. Jama, W.B. Saunders Co. 1983; 1834.
- Tietz NW, Fundamentals of Clin Chem. Young DS, Naito HK et al. 1973; 10: 79.
- Buccolo G and David M. Clin Chem 1973; 19: 476.
- Aykae G, Vysal M, Yalein AS , Kocak-Toker N, Sivas A. and Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, Glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985; 36 : 71–76.
- John Buege A. and Steven Aust D. Microsomal lipid peroxidation. London, Moury Kleiman Co. 1978; 302.
- Sing J and Reen RK. In vitro assessment of paracetamol-induced toxicity in the reuber hepatoma H4IIEC3/G cell line competent of xenobiotics metabolism. Toxicology in Vitro. 1999; 13 : 897–903.
- Rajesh MG and Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. Journal of Ethnopharmacology. 2004; 91: 99–104.
- Dortman RB and Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug and chemical toxicology. 1978; 1: 163–171.
- Premila Abraham.. Oxidative stress in paracetamol induced pathogenesis: (I) Renal damage. Indian J Biochem & Biophy. 2005; 42: 59–62.
- Affab Ahmad, Patil KK, Abul K, Najmi Shibli J, Ahmad, Pal SN and Balani DK. Evaluation of hepatoprotective potential of jigrine post treatment against thioacetamide induced hepatic damage. J Ethnopharmacol.2002; 70: 35–41.
- Thabew MI, Joice PD, TM and Rajatissa W. A comparative study of the efficacy of Pavetta indica and Osbekia octandra in the treatment of liver dysfunction. Planta Medica. 1987; 53: 239–241.
- Muriel P, Garciapina T, Perez-Alvarez V and Muelle M. Silymarin protect against paracetamol induced lipid peroxidation and liver damage. J Appl Toxicol. 1992; 12: 439–442.