Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nano-crystalline silver
- *Corresponding Author:
- Abhishek Adhya
Shanti Kunja Joraghat, Chinsurah , Hooghly - 712 101, West Bengal, India.
E-mail: abhishek0817@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Silver sulfadiazine (SSD) has been the standard topical antimicrobial for burn wounds for decades. Recently, nanometer‑sized silver particles are available which have high surface to volume ratio and remain effective even at a very low concentration and minimizes the chance for tissue toxicity due to silver. Hence, we conducted a randomized controlled trial to compare the effectiveness of topical SSD and nano‑crystalline silver (AgNP) hydrogel in burn wounds management. Materials and Methods: Study was conducted in the Burn Unit of IPGME&R; & SSKM Hospital Calcutta, from January 2011 to August 2012. Patients with 2° burn injury were randomly allocated to SSD and AgNP treatment group. Clinical assessments of burn wound were done on every week till 4th week and on completion of treatment. Results: Data for evaluation were obtained for 54 patients on SSD (2° deep‑dermal cases 27) and 52 (2° deep‑dermal cases 31) on AgNP treatment. Healing status of 2° deep‑dermal burns was more satisfactory for AgNP group than SSD treatment at 4 weeks. Among patients receiving AgNP, 80.6% showed at least 50% healing of 2° deep‑dermal wounds compared to 48.1% on SSD at 4 weeks (P = 0.001). The figures for complete healing at 4 weeks were, respectively, 4% and 0% (P = 0.116). Conclusions: AgNP can be an effective and superior alternative to SSD for burn wounds, particularly 2° deep‑dermal burns. Healing can be expected, in general, in 6 to 8 weeks time, depending upon the extent of body surface involvement.
Keywords
Burn wound assessment, nano-crystalline silver, silver sulfadiazine
Introduction
Burn is a common medico-surgical problem all over the world. It is probably the most devastating of all wounds, and, it imposes a serious burden on physical, mental, and socioeconomic conditions of the victim. It is estimated that, annually, about 11 million people over the world and 1 million people in India suffer from burn injuries. [1] According to a recent Indian study, mortality as high as 40.3% among 2499 burn patients was reported. [2]
Infection is a major problem for burn injuries as it delays the normal process of wound healing by prolonging the inflammatory phase of the immune response. Accumulation of dead tissue on the wound bed serves as rich nutrient source for bacteria, which, coupled with immunosuppression and exhaustion of body’s protein reserve enhances the chance of bacterial infection.
Silver sulfadiazine (SSD) has been the standard topical antimicrobial for burn wounds; however, it has some adverse effects such as argyria, leucopenia, hepatic, and renal toxicity. [3-6] Thus it demands a new therapy options for better burn wound management. Metallic silver holds a unique position as a strong antimicrobial agent to which resistance is not encountered. The advent of nanotechnology has permitted conversion of metallic silver into its fine nanoparticle form. These nano-sized silver particles are more effective than its pure form against microbial organisms and holds the promise of making topical silver therapy more effective and better tolerated. [7]
Hence, we have conducted this study to compare the clinical efficacy of compound silver (SSD) and nano-sized metallic silver (nano-crystalline: AgNP) among 2° burn wounds management.
Materials and Methods
Our study population comprised burn victims who were treated in a Burn Unit of a Tertiary Care Hospital at Kolkata, India. Period of study was from January 2011 to August 2012. The study was approved by the Institutional Ethics Committee, and informed consent was obtained from all patients during the study.
For the purpose of sample size calculation, the difference in duration of treatment for complete wound healing was considered as primary outcome measure. It was calculated that 64 subjects would be required per group in order to detect a difference of 5 days in this parameter with 80% power and 5% probability of type I error. This calculation assumed a standard deviation of 10 days for the complete wound healing parameter. From our earlier experience, 20% of subjects admitted to our burn unit are expected not to survive. Therefore, adjusting for dropouts, the recruitment target was kept at 80 subjects per group.
During this period, 106 patients aged between 5 and 60 years with 2° burn injury and 20–60% total body surface area (TBSA) involvement were recruited from among 244 screened. Patients with superficial (1°) or full-thickness (3°) burn injury, pregnancy, and significant co-morbidities such as preexisting cardiac disease, renal disease or diabetes were excluded.
The study was designed as an open-label, prospective, parallel group, randomized controlled trial. Simple randomization sequence was generated by computer software. After allocation of patients in two different groups, SSD and AgNP gel were administered topically on every alternate day in respective group. Totally, 163 subjects were randomized of whom data were analyzed for 54 patients treated with 1% SSD cream and 52 patients treated with AgNP gel. The assessment period was 4 weeks for each type of treatment. However, time taken for complete wound healing was also recorded.
Since the time required for complete wound healing is directly proportional to the extent of TBSA and depth of tissue injury involved, all patients in both treatment groups were divided into two groups that is, (i). 20–40% TBSA, (ii). 41–60% TBSA involvement groups; each of which were further sub-divided according to the depth of tissue injury as 2° superficial and 2° deep-dermal burns. Of 52 patients, 31 (59.62%) 2° deep-dermal cases from AgNP group and 27 (50%) 2° deep-dermal cases out of 54 on SSD were studied for the assessment of wound healing.
Condition of all ulcers/wounds was assessed at weekly intervals by examining: “(i) Edge of the ulcer/wound; (ii) Type of necrotic tissue present inside the ulcer/wound; (iii) Amount of necrotic tissue present inside the ulcer/wound; (iv) Color of skin surrounding the ulcer/wound; (v) Type of granulation tissue and its amount present inside the ulcer/wound; (vi) Amount of wound healing by means of epithelization of the ulcer/wound” – following photographic wound assessment tool (PWAT).[8,9] Besides various parameters in PWAT, (vii) Type of exudate inside the ulcer/wound; (viii) Amount of exudate inside the ulcer/wound; were also taken as determinants of burn wound healing, because, exudate increases the wound bio-burden and increases the need for dressing change, which, in turn, may delay wound healing.[10] These were, therefore, taken into account as well. The eight determinants of wound healing are depicted in Table 1. Assessors assigned a score between “0” and “4” for each determinant; the total score for each wound was calculated by summing up the scores assigned to the various determinants. Lesser score implied better wound condition. Percentage of improvement was calculated by applying the following formula: (initial score − final score)/initial score × 100. The outcome of treatment was categorized on the basis of percentage improvement as poor (0–25%), moderate (26–50%), good (51–75%), or excellent (76–100%).
| Score | Edge | Necrotic tissue type | Necrotic tissue amount % | Exudate type | Exudate amount | Skin color surrounding wound | Granulation tissue type and amount % | Epithelization % |
|---|---|---|---|---|---|---|---|---|
| 0 | Clearly visible | None | 0 | None | Dry | Pink/normal | Intact skin and 100 covered | 100 |
| 1 | Distinct but | White/ | >25 | Serous/clear | Just moist | Bright red | Beefy red and 75-<100 | 75-<100 |
| attached | nonadherent | covered | ||||||
| 2 | Not attached | Yellow | 25-<50 | Pale red | Small | White/ | Beefy red and 50-<75 | 50-<75 |
| slough | hypo-pigmented | covered | ||||||
| 3 | Rolled under | Adherent | 50-<75 | Bloody | Moderate | Dark red/purple | Pink/husky red and 25- | 25-<50 |
| <50 covered | ||||||||
| 4 | Fibrotic/ | Black | 75-100 | Purulent | Large | Black/ | None | <25 |
| scarred | escher | hyper-pigmented |
Individual items were summed up to obtain the burn wound score at a particular time point
Table 1: Determinants of burn wound assessment
Silver sulfadiazine 1% cream was purchased under the trade name of DISILVA Cream Diamond Drugs Pvt. Ltd (37, S.G Mullick Lane Kolkata-12. AgNP containing hydrogel – Carbopol 934 polymer was purchased from Loba Chemie Pvt. Ltd., India and the 0.5% w/v gel was prepared by dispersing specified amount of carbopol 934 powder into de-ionized water, mixing well and then leaving overnight to ensure complete swelling of the polymer [11] AgNP suspension, which was prepared by chemical reduction method using silver nitrate as precursor element, [12] was added to the material to a final concentration of 50 ppm. Finally, the gelling was done by drop-wise addition of triethanolamine in sufficient quantity till neutralization. The complete procedure was carried out aseptically; the product was packed in a sterile container and further subjected to UV sterilization.
Statistical analysis
Data have been summarized as mean ± standard deviation for numerical variables and counts and percentages for categorical variables. Numerical parameters have been compared between groups by Student’s independent samples t-test if normally distributed, or by Mann–Whitney U-test if otherwise. The Chi-square test for trend was used to compare the outcome of treatment between the study arms while the Fisher’s exact test was employed to compare proportions healed. Time trend toward 50% and complete healing have been studied by constructing Kaplan–Meier plots, which have been compared between the groups by log-rank test. Analysis was two-tailed, and P < 0.05 was considered statistically significant. Statistica version 6 (StatSoft Inc., 2001, Tulsa, Oklahoma, USA) and MedCalc version 11.6 (MedCalc Software 2011, Mariakerke, Belgium) software were used for analysis.
Results
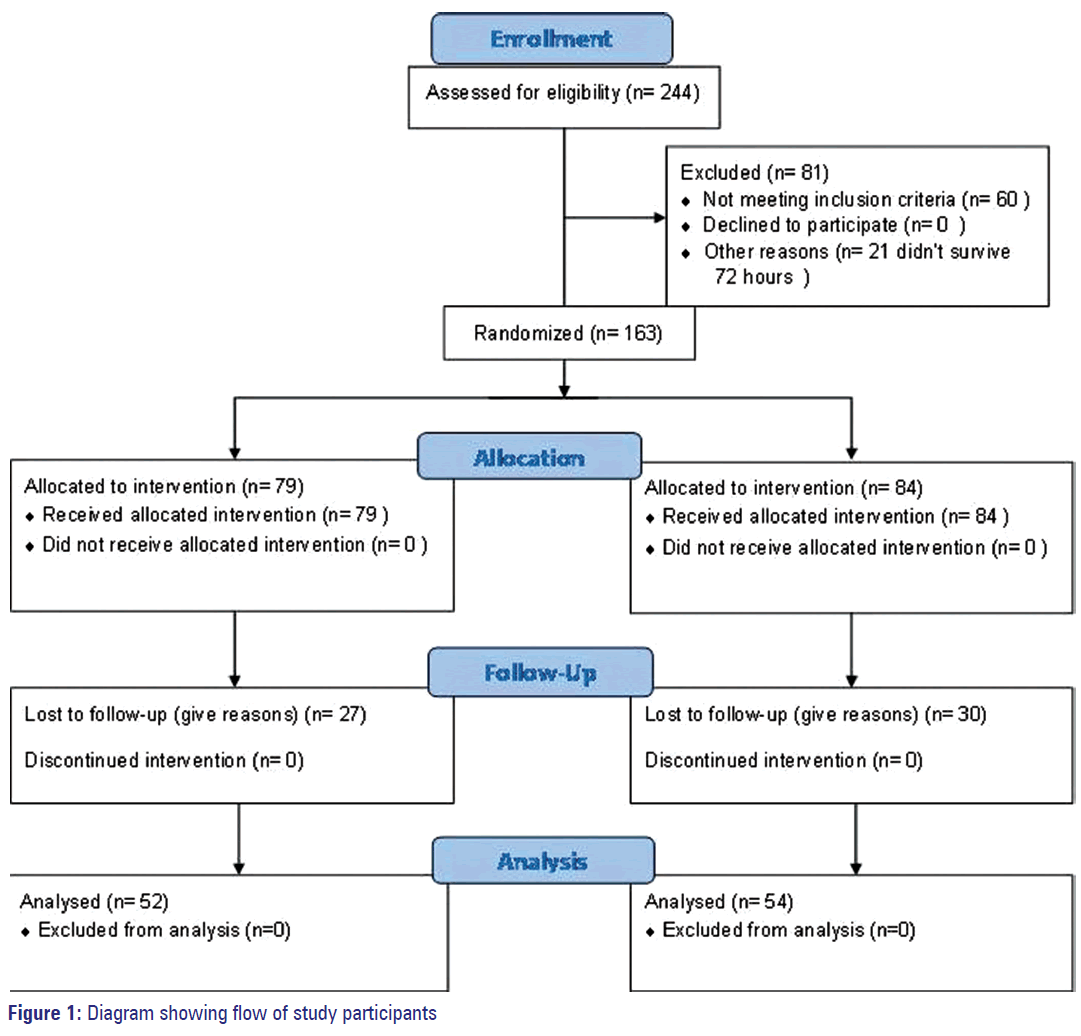
The flow of study participants is depicted in Figure 1. Of the 52 patients recruited into AgNP group 25 were males, and among 54 patients in SSD group 29 were males. Patients in the former group ranged between 7 and 60 years in age, with mean and SD of 27.4 ± 11.34 years. Age range in the latter group was 12-55 years with mean and SD of 31.8 ± 10.66. As shown in Table 2, there was no statistically significant difference between groups in age and gender distribution. The extent of body surface area burnt was also comparable between the groups.
| Parameter | Silver sulfadiazine (n=52) (%) | Nano-silver (n=54) (%) | P |
|---|---|---|---|
| Age | 31.8±10.66 | 27.4±11.34 | 0.064 |
| Sex | |||
| Male | 29 (53.70) | 25 (48.08) | 0.697 |
| Female | 25 (46.30) | 27 (51.92) | |
| Total body surface area burnt (%) | |||
| 20-40 (2° superficial) | 12 (31.48) | 15 (28.85) | 0.992 |
| 20-40 (2° deep-dermal) | 13 (24.07) | 17 (32.69) | |
| >40-60 (2° superficial) | 10 (18.52) | 6 (11.54) | |
| >40-60 (2° deep-dermal) | 14 (25.92) | 14 (26.92) |
P value in the last column is from Independent samples t-test for age, Fisher’s exact test for gender and Chi-square test for body surface area involvement
Table 2: Demographics and clinical data of burn patients in both treatment groups
Data in Table 3 depict the average time required for complete wound healing among various categories of burn wounds. The differences between groups were statistically significant for deep-dermal wounds only, with patients in the AgNP arm recovering on average 10 days earlier than in their SSD counterparts when body surface area involved was between 20% and 40% and 13 days earlier when involvement was >40–60%.
| Group | Total body surface area burnt | |||
|---|---|---|---|---|
| 20-40% | >40-60% | |||
| 2° superficial | 2° deep-dermal | 2° superficial | 2° deep-dermal | |
| Silver sulfadiazine (n=52) | 20.5±8.75 (n=17) | 48.4±14.11 (n=13) | 28.1±12.76 (n=10) | 58.9±18.18 (n=14) |
| Nano-silver (n=54) | 15.7±4.14 (n=15) | 38.6±11.26 (n=17) | 26.0±6.22 (n=6) | 45.4±11.35 (n=14) |
| P | 0.206 | 0.022 | 0.739 | 0.007 |
P value is from between group comparison by Mann-Whitney U-test
Table 3: Duration of treatment for complete wound healing (days) by wound type
As shown in Table 4, considering deep-dermal burn wounds only, the differences in treatment outcome at 4 weeks was statistically highly significant (P = 0.003) in favor of AgNP treatment. However, at 4 weeks, only 4 cases in AgNP arm had achieved complete wound healing compared to none in the SSD arm, and this was not a statistically significant difference [Table 5]. However, 25 had achieved 50% wound healing compared to 13 on SSD, and this was statistically significant (P = 0.001).
| Group | Poor | Moderate | Fast | Excellent | P |
|---|---|---|---|---|---|
| (0-25%) | (26-50%) | (51-75%) | (76-100%) | ||
| Silver sulfadiazine | 6 | 8 | 13 | 0 | 0.003 |
| (n=27) | |||||
| Nano-silver (n=31) | 2 | 4 | 20 | 5 |
P value is from between group comparison by Chi-square test for trend
Table 4: Comparison of healing status of 2° deepdermal burn wounds after 4 weeks treatment
| Group | 50% healing by 4 weeks (%) | Complete healing by 4 weeks (%) | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Silver sulfadiazine (n=27) | 13 (48.14) | 14 (51.85) | 0 (0) | 27 (100) |
| Nano-silver (n=31) | 25 (80.64) | 6 (19.35) | 4 (12.9) | 27 (87.09) |
| P | 0.001 | 0.116 | ||
P value is from between group comparison by Fisher’s exact test
Table 5: Status of wound healing (50% healing or complete healing) by 4 weeks
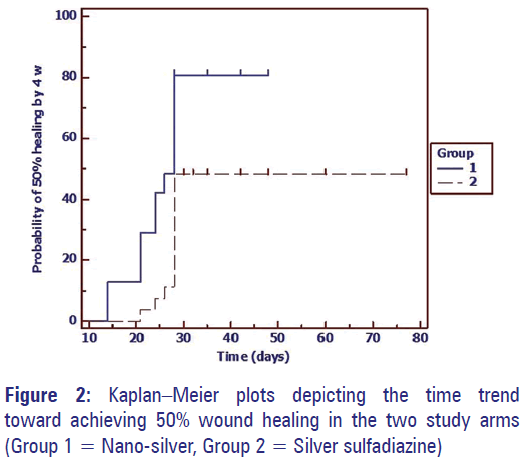
The probability of achieving 50% wound healing by 4 weeks have been depicted in the Kaplan–Meier plots in Figure 2. The log-rank test also indicates significantly faster achievement of this endpoint in the AgNP group compared to the SSD group (P = 0.001).
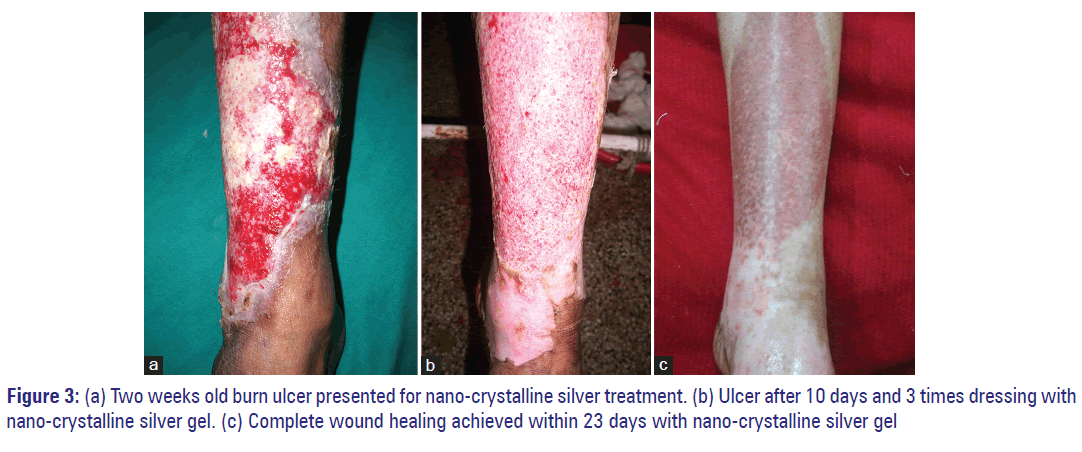
Photograph of a clinical case, who was treated with nano silver (AgNP), is shown here as an example to describe how we have clinically scored the burn ulcer and what was the response of the therapy. Figure 3a shows a 35-year-old male patient admitted with 43% TBSA flame burn, with an ulcer in right lower limb, the ulcer had fibrotic edge (score: 4), with <50% (score: 2), adherent necrotic (score: 3) tissue, with moderate (score: 3) bloody (score: 3) exudate, with surrounded hypo-pigmented (score: 2) skin, with husky red (score: 3) <25% granulation, with <25% (score: 4) epithelization, with a total score 24/40. The patient was treated in a district hospital for 2 weeks, but the wound was not improving and transferred to our burn unit. After admission in our center, we started dressing with nano-silver (AgNP). Within 10 days the patient shows significant clinical improvement [Figure 3b] and within 23 days, patient had complete wound healing [Figure 3c].
Both the topical treatments were well-tolerated and apart from mild irritation during the application in a few subjects; no other adverse event was noticed. There were no instances of argyria as well.
Discussion
Effective healing of skin wounds implies the regeneration of normal skin by resurfacing with new epithelium. In the case of superficial burns, when epidermis is destroyed leaving dermis and its elements intact, the skin virtually restores its epidermal cover within 7–14 days without any complication. However, in 2° burn, only a part of dermis remains viable, and wounds take several weeks to heal. The healing process may be retarded by disintegrative necrosis of the upper dermis which is susceptible to bacterial infection. As a deep-dermal burn heals slowly, the chance for infection is more prolonged than superficial burns. In the addition, formation of necrotic slough provides a rich source of nutrition that enhances bacterial growth. [13]
A number of silver compounds with antimicrobials activity are used prophylactically to prevent infective complications in burn wounds. Among them, mafenide acetate 11.2% cream (e.g., sulfamylon) is one of the oldest effective topical antimicrobial agents; it has broad spectrum of antimicrobial activity, with some antifungal properties and has good penetration through the eschar. But, mafenide cream is toxic to epithelial cells and fibroblasts and it can cause an allergic skin rash and being a carbonic anhydrase inhibitor, it can also cause metabolic acidosis, so it is not preferred as a first-line antimicrobials ointment for burn wounds. Unlike mafenide or silver nitrate, SSD does not hinder epithelialization, although it does hamper contraction of fibroblasts. Furthermore, SSD is painless on application, has high patient acceptance, and is easy to use with or without dressing. [14] Thus, SSD is widely used and has been considered as the “gold standard” topical treatment for burn wounds and, therefore, it was selected as the active comparator in our study. However, it is not the ideal topical antimicrobial, because deep-dermal wounds usually heal slowly and there is suspicion that SSD may delay the process further, [4,15,16] and bacterial resistance to SSD has also been reported. [4,17] The sticky nature of the preparation can make the periodic dressing change process painful. Leukopenia, hepatic, and renal toxicity have also been documented following prolonged application of SSD. [4,5] Thus there is a need to continue the search for a superior antimicrobial with desirable effects on burn wound healing.
In recent years, with the advent of nanotechnology, pure form of silver can now be utilized as a topical treatment for burn wounds. The nanometer-sized particles are metastable in nature and thus more reactive than bulk form. [18,19] The high surface to volume ratio of the nanoparticles is another advantage as it allows the particles to remain effective even at a very low concentration. Thus minimizes the chance for tissue toxicity, if any. Several studies have reported a broad spectrum antibacterial as well as antifungal properties of nano-crystalline silver. [17,20,21] The mechanism may involve disruption of bacterial cell wall, blocking of DNA replication and deactivation of vital enzymes of bacterial respiratory system. Thus, nano-crystalline silver can be an effective barrier against microbial invasion and significantly decrease the risk of infection.
In addition to the superior antimicrobial action, AgNP has potential antiinflammatory effects, less tissue toxicity, analgesic property and faster wound healing by achieving moist condition under a scab and there is already evidence to suggest that AgNP formulations can achieve better wound outcome than conventional silver preparations, and this may be in ways beyond just excellent antimicrobial action. [17,22-27]
Our results suggest that significantly faster and improved wound healing can be achieved by topical application of nano-crystalline silver (AgNP) as compared to conventional SSD. This is particularly true of 2° deep-dermal wounds. However, complete wound healing time extends beyond 4 weeks and generally requires 6–8 weeks or even more. This is in agreement with earlier studies. [13] No adverse effects were encountered, and there was no instance of argyria.
To conclude, nano-crystalline silver (AgNP) can be an effective and superior alternative to SSD in the management of burn wounds, particularly 2° deep-dermal burns. Healing can be expected, in general, in 6–8 weeks time, depending upon the extent of body surface involvement. Confirmation of these results in larger trials, with exploration of pharmacoeconomic aspects, will be worthy areas for future study.
Acknowledgement
We are grateful to Department of Science & Technology West Bengal for providing financial resources and also to the Medical Superintendent cum Vice-Principal, IPGME&R; SSKM Hospital & The West Bengal University of Health Sciences for academic support.
References
- World Health Organization. BURNS Fact sheet No 365. Geneva. Available from: http://www.who.int/mediacentre/factsheets/fs365/en/. [Last updated on 2014 Apr Last cited on 2014 Jul 06].
- Bain J, Lal S, Baghel VS, Yedalwar V, Gupta R, Singh AK. Decadorial of a burn center in Central India. J Nat Sci Biol Med 2014;5:116-22.
- Abedini F, Ahmadi A, Yavari A, Hosseini V, Mousavi S. Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy. Int Wound J 2013;10:573-8.
- Muller MJ, Hollyoak MA, Moaveni Z, Brown TL, Herndon DN, Heggers JP. Retardation of wound healing by silver sulfadiazine is reversed by Aloe vera and nystatin. Burns 2003;29:834-6.
- Chaby G, Viseux V, Poulain JF, De Cagny B, Denoeux JP, Lok C. Topical silver sulfadiazine-induced acute renal failure. Ann Dermatol Venereol 2005;132:891-3.
- Fraser JF, Cuttle L, Kempf M, Kimble RM. Cytotoxicity of topical antimicrobial agents used in burn wounds in Australasia. ANZ J Surg 2004;74:139-42.
- Kingensmith EM. The Washington Manual of Surgery. 5th ed.. 21st May 2008: Lippincott Williams & Wilkins. Washington University (Saint Louis, Mo.). p. 128.
- Houghton PE, Kincaid CB, Campbell KE, Woodbury MG, Keast DH. Photographic assessment of the appearance of chronic pressure and leg ulcers. Ostomy Wound Manage 2000;46:20-6, 8.
- Hughes AC. Photographing wounds. J Wound Care 1995;4:314-7.
- Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D’Souza L. MEASURE: A proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12 3 Suppl: S1-17.
- Mekkawy A, Fathy M, El-Shanawany S. Formulation and in vitro Evaluation of fluconazole topical gels. Br J Pharm Res 2013;3:293-313.
- Janardhanan R, Karuppaiah M, Hebalkar N, Rao TN. Synthesis and surface chemistry of nano silver particles. Polyhedron 2009;28:2522-30.
- Sevitt S. Burns: Pathology and Therapeutic Applications. London: Butterworth & Co. Ltd.; 1957. p. 54-74.
- Klein MB. Thermal, chemical, and electrical injuries. In: Thorne CH, Beasley RW, Aston SJ, Bartlett SP, Gurtner GC, Spear SL, editors. Grabb and Smith’s Plastic Surgery. 6th ed.. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins; 2006. p. 132-49.
- Hosseinimehr SJ, Khorasani G, Azadbakht M, Zamani P, Ghasemi M, Ahmadi A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol Croat 2010;18:2-7.
- Khorasani G, Hosseinimehr SJ, Azadbakht M, Zamani A, Mahdavi MR. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg Today 2009;39:587-91.
- Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007;33:139-48.
- Pradeep T. A Textbook of Nanoscience and Nanotechnology. New Delhi: Tata McGraw Hill Edu. Pvt. Ltd.; 2012. p. 205-8.
- Sau TK, Rogach AL. Complex-Shaped Metal Nanoparticles: Bottom-Up Syntheses and Applications. 1st ed.. Boschstr. 12, 69469 Weinheim, Germany: Willey-VCH; 2012. p. 215-35.
- Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol 2010;44:5649-54.
- Wong KK, Liu X. Silver nanoparticles-the real “silver bullet” in clinical medicine? Med Chem Commun 2010; 1:125-31.
- Wong KK, Cheung SO, Huang L, Niu J, Tao C, Ho CM, et al. Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem 2009;4:1129-35.
- Vaidyanathan R, Kalishwaralal K, Gopalram S, Gurunathan S. Nanosilver – the burgeoning therapeutic molecule and its green synthesis. Biotechnol Adv 2009;27:924-37.
- Fong J, Wood F. Nanocrystalline silver dressings in wound management: A review. Int J Nanomedicine 2006;1:441-9.
- Leaper DJ. Silver dressings: Their role in wound management. Int Wound J 2006;3:282-94.
- Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293-4.
- Atiyeh BS, Al-Amm CA, El-Musa KA, Sawwaf A, Dham R. The effect of moist and moist exposed dressings on healing and barrier function restoration of partial thickness wounds. Eur J Plast Surg 2003;26:5-11.