Formulation and in vitro evaluation of Hydrodynamically balanced system for theophylline delivery
- *Corresponding Author:
- Amit Kumar Nayak
Department of Pharmaceutics, Seemanta Institute of Pharmaceutical Sciences, Mayurbhanj, Orissa, India
E-mail: amitkrnayak@yahoo.co.in
Date of Received: 13-05-2011
Date of Accepted: 20-06-2011
Available Online: 15-08-2011
Abstract
The objective of the present study was to formulate hydrodynamically balanced systems (HBSs) of theophylline as single unit capsules. They were formulated by physical blending of theophylline with hydroxypropyl methyl cellu-lose, polyethylene oxide, polyvinyl pyrrolidone, ethyl cellulose, liquid paraffin, and lactose in different ratios. These theophylline HBS capsules were evaluated for weight uniformity, drug content uniformity, in vitro floating behavior and drug release in simulated gastric fluids (pH 1.2). All these formulated HBS capsules containing theophylline were floated well over 6 hours with no floating lag time, and also showed sustained in vitro drug release in simulated gas-tric fluid over 6 hours. The theophylline release from these capsules was more sustained with the addition of release modifiers (ethyl cellulose and liquid paraffin). The drug release pattern from these capsules was correlated well with first order model (F-1 to F-5) and Korsmeyer-Peppas model (F-6 and F-7) with the non-Fickian (anomalous) diffusion mechanism. These experimental results clearly indicated that these theophylline HBS capsules were able to remain buoyant in the gastric juice for longer period, which may improve oral bioavailability of theophylline.
Keywords
Hydrodynamically balanced system; gastroretention; drug release; hydrocolloids; theophylline; capsules
Introduction
Oral administration of dosage forms is the most convenient and preferred means of drug delivery to the systemic circulation. Many attempts have been made to develop sustained release formulations withextended clinicaleffects with reduced dosing frequency. Oral sustained drug delivery formulations show some limitations related with the gastric emptying time. The variable and too rapid gastrointestinal transit could result in incomplete drug release from the dosage form at the absorption site in gastrointestinal tract lading to weaken efficacy of the administered dose [1]. Prolonged gastric residence of dosage forms increase duration of drug release, improve drug solubility in gastric pH environment and reduce drug waste with improved bioavailability [2]. Over the last two decades, several gastroretentive drug delivery approaches being designed and developed to prolong gastric residence time, viz. floatation, mucoadhesion, sedimentation, unfoldable, expandable, or swellable systems, superporous hydrogel systems, magnetic systems etc [3-4]. Floating dosage forms are designed to be remained buoyant in the gastric juice, thus retained in the stomach for several hours [2,5].
Among various gastroretentive floating systems, hydrodynamically balanced systems (HBSs) have gained a lot of significance in recent days to increase gastroretention, which improve absorption of drugs especially those are absorbed from stomach and small intestine [6-7]. These systems contain drug with gelforming hydrocolloids meant to remain buoyant for several hours in the stomach content. On contact with the gastric fluid, these systems form a water colloidal gel barrier around their surface and maintain a bulk density less than 1. They are mainly single-unit dosage forms, and usually composed of one or more gel-forming hydrophilic polymeric substances and an active pharmaceutical ingredient [8]. Various gastroretentive HBSs for few drug candidates have already investigated [6-11].
Theophylline (3, 7-Dihydro-1, 3-dimethylpurine-2, 6(1H)-dione; 1, 3-Dimethylxanthine), a xanthine derivative bronchodilator, is used to treat both chronic and acute asthmatic attacks. It is also used in the management of chronic obstructive pulmonary disease. The major drawback of orally administered dosage form of theophylline is its short half-life (6 hours), and it requires frequent dosing for achieving therapeutic drug concentration in the target site [12]. But, sustained delivery of theophylline may provide desirable serum concentrations for prolonged periods without frequent dosing, thereby providing patient compliance. In previous literature, only one hydrodynamically balanced system (HBS)-based capsule for floating delivery of theophylline was reported [13]. Hydroxypropyl methyl cellulose, methyl cellulose, hydroxyethyl cellulose, sodium carboxymethyl cellulose, ethyl cellulose, glyceryl monostearate, dibasic calcium phosphate, mannitol, tragacanth were used for the preparation of these floating capsules. In the present investigation, we made an attempt to prepare theophylline HBS capsules using hydroxypropyl methyl cellulose (HPMC K4M), polyvinyl pyrrolidone K30 (PVP K30), ethyl cellulose, polyethylene oxide (PEO 60 K), liquid paraffin, and lactose to control the delivery of theophylline for longer period in stomach with minimum floating time of 6 hours.
Materials and Methods
Materials
Theophylline and polyethylene oxide (PEO 60 K) were obtained from B. S. Traders Pvt. Ltd., India. Hydroxypropyl methylcellulose (HPMC K4M), polyvinyl pyrrolidone K30 (PVP K30), and ethyl cellulose were purchased from Loba Chemie Pvt. Ltd., India. Liquid paraffin (Nice Chemicals Pvt. Ltd, India) and lactose (S.D. Fine Chemicals Ltd., India) were used. All other chemicals and reagents used were of analytical grade.
Preparation of theophylline HBS capsules
Theophylline HBS capsules were prepared by simple blending of 100 mg of theophylline and selected different polymers. Magnesium stearate (5 % w/w) as lubricating agent was added to the blend and mixed for additional 3 minutes. The final blend was filled into empty hard gelatin capsules (size 0) manually. Care was taken to fill the contents completely to maintain the uniformity of contents and weight. The composition of the HBS capsules is given in Table 1.
| Components | Formulation Codes | ||||||
|---|---|---|---|---|---|---|---|
| F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 | |
| Theophylline (mg) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| HPMC K4M (mg) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PEO 60 K (mg) | 75 | 65 | 55 | 65 | 55 | 72 | 69 |
| PVP K 30 (mg) | − | 10 | 20 | − | − | − | − |
| Ethyl cellulose (mg) | − | − | − | 10 | 20 | − | − |
| Liquid paraffin (mg) | − | − | − | − | − | 3 | 6 |
| Lactose (mg) | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Magnesium stearate (%) | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
Table 1: Composition of various theophylline HBS capsules
Determination weight uniformity
To determine capsule weight uniformity, 30 capsules were sampled and accurately weighed using an electronic analytical balence. The results were expressed as mean values of 30 determinations. The coefficient of variation was calculated using the formula:
Coefficient of variation (%) = Standard deviation / Mean × 100
Determination of drug content
The theophylline HBS capsules of each formulation were dissolved in 0.1 N HCl. Samples were filtered using Whatmann filter (Grade I) paper and then, used for determination of theophylline contents by using a UV-VIS spectrophotometer (U. V. 2440 Double beam spectrophotometer, SHIMADZU Corporation, Japan) at 271 nm. Th ree replicate determinations were made for each formulation.
In vitro floating properties
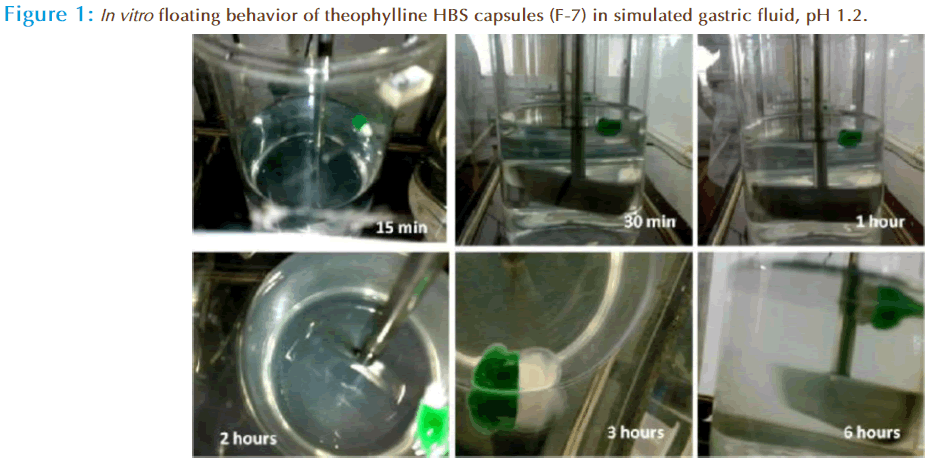
The capsules were immersed in 900 ml of in simulated gastric fluid, pH 1.2 in USP type II apparatus at 50 rpm maintained at 37 ± 5°C for 6 hours. The time for which the capsules constantly remain float on the surface of the medium (buoyant) was observed visually and was taken as the floating time.
In vitro drug release studies
In vitro drug release studies were carried in USP type II apparatus at 50 rpm maintained at 37 ± 5°C. Various theophylline capsules were placed into the dissolution medium of 900 ml of simulated gastric fluid (pH 1.2). The 5 ml of aliquots was withdrawn from the dissolution vessel at specific time intervals and replaced with equivalent volume of fresh medium. Collected dissolution samples were filtered using Whatmann filter (Grade I) paper and then, used for determination of released theophylline concentrations by using a UV-VIS spectrophotometer (U. V. 2440 Double beam spectrophotometer, SHIMADZU Corporation, Japan) at 271 nm. Each in vitro release study was performed in triplicate.
Analysis of release data
To analyze the mechanism of drug release from these theophylline capsule matrices, the in vitro dissolution data were fitted to various mathematical models like zero order, first order, Higuchi, and Korsmeyer-Peppas models [14-17].
Zero-order Model: F = K0 t, where F represents the fraction of drug released in time t, and K0 is the apparent release rate constant or zero-order release constant.
First-order Model: ln (1-F) = - K1st t, where F represents the fraction of drug released in time t, and K1 is the first–order release constant.
Higuchi Model: F = KH t½, where F represents the fraction of drug released in time t, and KH is the Higuchi dissolution constant.
Korsmeyer-Peppas Model: F = KP tn, where F represents the fraction of drug released in time t, KP is the rate constant and n is the release exponent, this indicates the drug release mechanism.
Again, the Korsmeyer-Peppas model has been employed in the in vitro drug release behavior analysis of various pharmaceutical formulations to distinguish between various release mechanisms: Fickian release (diffusion-controlled release), non-Fickian release (anomalous transport), and case-II transport (relaxation- controlled release). When, n ≤ 0.5, it is Fickian release. Then value between 0.5 and 1.0 is defined as non-Fickian release. When, n ≥ 1.0, it is case-II transport and this involves polymer dissolution and polymeric chain enlargement or relaxation [17].
Mean dissolution time (MDT) is used to characterize the drug release rate from a dosage form and indicates the drug release-retarding efficiency of the polymer. In this study, MDT was calculated from dissolution data using the following formula [18]:
MDT = [n/n + 1] × KP(-1/n),
where n is the release exponent and KP is the release rate constant.
Results and Discussion
Drug release from hard gelatin capsules containing polymers and other excipients is commonly used in oral drug delivery due to their easy production facility, flexibility to obtain a desirable drug release profile, cost-effectiveness, and broad regulatory acceptance [19-21]. Various excipients along with hydrocolloid polymers, release modifiers, and theophylline were filled into hard gelatin capsules to prepare HBS of theophylline (size 0), and evaluated for their in vitro performances.
The result of weight uniformity of these theophylline capsules is shown in Table 2. All these formulations met the USP specifications for weight uniformity. The mean weight of these formulated theophylline HBS capsules varied from 339.34 ± 6.85 to 342.73 ± 6.11 mg. The coefficient of variation of these capsules varied from 1.51 to 2.18 %. The values of coefficient of weight variations indicate that filling of capsules carried out properly. The drug content (%) within these theophylline capsules were determined and this was within the range between 97.66 ± 3.68 to 99.02 ± 3.05 % (Table 2). This result confirms that the uniform mixing of theophylline with other ingredients.
| Formula- tion code | Weight uniformity | Drug content (%) ± S.D.* | ||
|---|---|---|---|---|
| Mean weight ± S.D.* (mg) | Coefficient of variation (%) † | |||
| F-1 | 342.03 | ± 7.47 | 2.18 | 98.36 ± 2.45 |
| F-2 | 341.87 | ± 7.04 | 2.06 | 99.02 ± 3.05 |
| F-3 | 341.32 | ± 5.15 | 1.51 | 97.97 ± 3.12 |
| F-4 | 340.18 | ± 6.88 | 2.02 | 98.52 ± 2.93 |
| F-5 | 342.73 | ± 6.11 | 1.78 | 97.66 ± 3.68 |
| F-6 | 340.16 | ± 6.92 | 2.03 | 98.70 ± 3.16 |
| F-7 | 339.34 ± 6.85 | 2.02 | 98.95 ± 4.02 | |
Table 2: Weight uniformity and drug content (%) results of theophylline capsules
All these HBS capsules containing theophylline were evaluated for in vitro floating behavior in 900 ml of in simulated gastric fluid, pH 1.2 in USP type II apparatus at 50 rpm maintained at 37 ± 5°C for 6 hours. They were floated well with no floating lag time. The floating time of these theophylline HBS capsules were more than 6 hours (Figure 1). This may be accounted to increased gel strength of the polymeric combination matrices with drug in the form of HBS capsules. The mechanism involved in buoyancy of these HBS capsules, could be rapid hydration and swelling of the polymeric matrices producing a floating mass in the gastric pH (1.2). The swelling of the polymeric chains could be increased with an increase of polymer viscosity, but the highly viscous polymers formed a consistent hydrogel that could block the solvent’s deeper penetration into the core of the HBS. The matrix integrity of these HBS capsules was satisfactory.
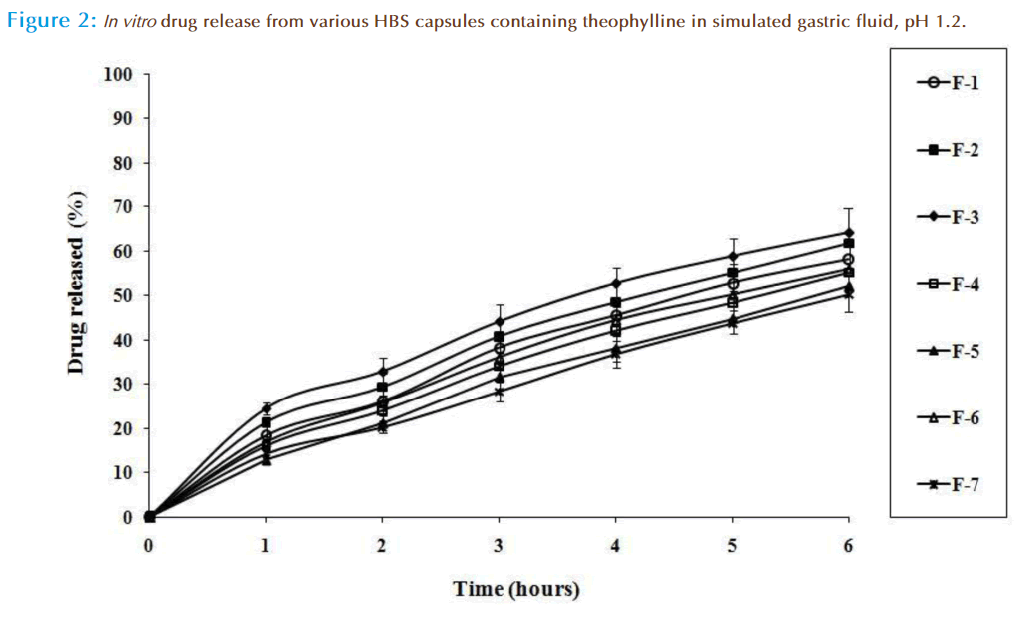
In vitro drug release studies were carried out in in simulated gastric fluid (pH 1.2), as dissolution medium. All these HBS capsules containing theophylline showed sustained drug release over 6 hours (Figure 2). From the results of in vitro drug release study, MDT values in simulated gastric fluid (pH 1.2) for all formulated HBS capsules were calculated and presented in Table 3. MDT value was used to characterize the drug release rate from formulations, and release retarding efficiency of formulations. The MDT value of the theophylline HBS capsules containing liquid paraffin was higher (6.78 and 5.60 hours for F-6 and F-7, respectively) in comparison with other HBS capsules formulated in this investigation, which indicates the comparatively higher drug release retarding efficiency than that of others. But, the theophylline HBS capsules containing PVP K 30 experienced comparatively lower MDT values (5.03 and 4.68 hours, for F-2 and F-3, respectively). Again, it was observed that the addition of ethyl cellulose and liquid paraffin as release modifier, the drug release from these theophylline capsules was retarded. But, the drug release from these HBS capsules was increased with increasing amount of PVPK 30. The drug release from these HBS capsules was increasing with the addition of PVP K 30 due to less viscosity of its than HPMC K4M and PEO 60 K. In our previous report, we also found the sustained release behaviour of drug from HBS capsules containing liquid paraffin [22]. In agreement with this work, it was observed that the liquid paraffin as release modifier produced comparatively more sustained theophylline release from these HBS capsules.
The higher viscosity due to presence of HPMC K4M as hydrocolloid polymer in these theophylline HBS capsules may promote the formation of highly viscous gels upon contact with aqueous fluids. This would retard the theophylline release rate from these HBS capsules containing HPMC K4M. In a parallel line, Siepmann and Peppas suggested that the drug release from hydroxypropyl methylcellulose matrices is sequentially governed as follows [23]: (i) At the beginning, steep water concentration gradients are formed at the polymer/water interface resulting in water imbibition into the matrix; (ii) Due to the imbibition of water, hydroxypropyl methylcellulose swells resulting in dramatic changes of polymer, drug concentrations and increasing dimensions of the system; (iii) Upon contact with water, the drug dissolves and diffuses out of the device due to concentration gradient; and (iv) With increasing water content, the diffusion coefficient of the drug increases substantially. In addition with HPMC K4M, PEO 60 K was incorporated in various theophylline HBS capsules. HPMC K4M and PEO 60 K have been used for modulating drug release and to prevent the burst release of highly soluble drug molecules [24-25]. It is reported that polyethylene oxide has been used in hydroxypropyl methylcellulose matrices to achieve expanded swelling, resulting in enhanced gastroretention of the sustained release dosage forms [25]. In addition, it is also reported that the high-swelling capacity of water-soluble polymer polyethylene oxide has been extensively used as a controlled release excipient to modify drug release and dissolution from solid hydrophilic matrix preparations [26]. Once in contact with a liquid, polyethylene oxide will start to hydrate and swell, forming a hydrogel layer that regulates further penetration of the liquid into the matrix and the diffusion of the drug molecules from the dosage form. As a result of hydrogel formation, the rate of water intake slows down while those of drug release declines and prolongs.
To analyze the mechanism of drug release from these theophylline HBS capsules, the in vitro dissolution data were fitted to various mathematical models like zero order, first order, Higuchi, and Korsmeyer-Peppas models. The results of the curve fitting into these above-mentioned mathematical models are given in Table 4. The drug release pattern of HBS capsules of formulation F-1 to F-5 was correlated well with first order model (R2 = 0.9951 to 0.9974) over a period of 6 hours. On the other hand, other formulations, F-6 and F-7 were correlated with Korsmeyer-Peppas model (R2 = 0.9972 and 0.9958, respectively) over a period of 6 hours, when their respective correlation coefficients in simulated intestinal fluid were compared.
| Formulation code | MDT (hours) |
|---|---|
| In simulated gastric fluid | |
| F-1 | 5.36 |
| F-2 | 5.03 |
| F-3 | 4.68 |
| F-4 | 6.27 |
| F-5 | 6.52 |
| F-6 | 5.60 |
| F-7 | 6.78 |
Table 3: Mean dissolution time (MDT) of theophylline HBS capsules in simulated gastric fluid, pH 1.2.
| Code | Zero order | First order | Higuchi | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | K0(Hour-1) | R2 | K1st(Hour-1) | R2 | KH(Hour-1) | R2 | KP(Hour-1) | |
| F-1 | 0.9838 | 0.0826 | 0.9951 | 0.1391 | 0.9800 | 0.2879 | 0.9896 | 0.1761 |
| F-2 | 0.9877 | 0.0831 | 0.9957 | 0.1477 | 0.9829 | 0.2893 | 0.9813 | 0.2020 |
| F-3 | 0.9815 | 0.0820 | 0.9958 | 0.1543 | 0.9847 | 0.2865 | 0.9754 | 0.2343 |
| F-4 | 0.9734 | 0.0786 | 0.9974 | 0.1260 | 0.9842 | 0.2728 | 0.9870 | 0.1548 |
| F-5 | 0.9818 | 0.0780 | 0.9971 | 0.1177 | 0.9735 | 0.2709 | 0.9882 | 0.1274 |
| F-6 | 0.9886 | 0.0786 | 0.9660 | 0.1186 | 0.9804 | 0.2739 | 0.9972 | 0.1676 |
| F-7 | 0.9762 | 0.0746 | 0.9710 | 0.0974 | 0.9721 | 0.2570 | 0.9958 | 0.1322 |
Table 4: Results of curve fitting of the in vitro theophylline release data from different theophylline HBS capsules matrices in simulated gastric fluid, pH 1.2.
The Korsmeyer-Peppas model has been also employed in the in vitro drug release behavior analysis of various pharmaceutical formulations to distinguish between various competing release mechanisms: Fickian release (diffusion-controlled release), non-Fickian release (anomalous transport), and case-II transport (swelling- controlled release). The value of release exponent (n) determined from in vitro theophylline release data of various HBS capsules ranged from 0.5670 to 0.7589 in simulated gastric fluid (Table 5), which indicated anomalous (non-Fickian) diffusion. This could be attributed to the high water uptake by these HBS capsules containing hydrocolloids leading to higher swelling of these capsules supported the anomalous release mechanism of theophylline.
| Formulation code | Release exponent (n) | Release mechanism |
|---|---|---|
| F-1 | 0.6697 | non-Fickian |
| F-2 | 0.6215 | non-Fickian |
| F-3 | 0.5670 | non-Fickian |
| F-4 | 0.7030 | non-Fickian |
| F-5 | 0.7589 | non-Fickian |
| F-6 | 0.6800 | non-Fickian |
| F-7 | 0.7281 | non-Fickian |
Table 5: Release exponent and release mechanism of various theophylline HBS capsules obtained from in vitro drug release study.
Conclusion
Gastroretentive theophylline HBS capsules were prepared using hydroxypropyl methyl cellulose, polyethylene oxide, polyvinyl pyrrolidone, ethyl cellulose, liquid paraffin, and lactose to control the delivery of theophylline for longer period in stomach with minimum floating time of 6 hours. All these formulated HBS capsules were floated well over 6 hours with no floating lag time. They also showed sustained drug release in simulated gastric fluid, pH 1.2 over 6 hours. The theophylline release from these HBS capsules was more sustained with the addition of ethyl cellulose and liquid paraffin as release modifiers. The drug release pattern of these theophylline HBS capsules was correlated well with first order model (F-1 to F-5) and Korsmeyer-Peppas model (F-6 and F-7) with the non-Fickian (anomalous) diffusion mechanism. The results of current study clearly indicate a promising potential of these HBS capsules containing theophylline as an improved alternative to its sustained release dosage forms. However, further clinical studies are needed to assess the utility of this HBS system.
References
- Iannuccelli V, Coppi G, Bernabei MT, et al. Air compartment multiple-unit system for prolonged gastric residence. Part I. Formulation study. Int J Pharm. 1998; 174: 47-54.
- Sing BN and Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 2000; 63: 235-59.
- Nayak AK, Majir and Das, B. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010; 3(1): 2-10.
- Nayak AK, Malakar J and Sen KK. Gastroretentive drug delivery technologies: Current approaches and future potential. J Pharm Educ Res. 2010; 1(2): 1-12.
- Arrora S, Ali J, Khar RK, et al. Floating drug delivery systems: A review. AAPS PharmSciTech. 2005; 6(3): 372-90.
- Sahni JK, Ahmad FJ, Ahuja A, et al. Formulation and evaluation of a hydrodynamically balanced system of paracetamol. The Indian Pharmacist. 2006; V (46): 64-66.
- Seth PR and Tossounian J. The hydrodynamically balanced system, a novel drug delivery system for oral use. Drug Dev Ind Pharm. 1984; 10: 313-39.
- Dorozynski P, Kulinowski P, Jachowicz R, et al. Development of a simultaneous studies and magnetic resonance imaging of water transport in hydrodynamically balanced systems: A technical note. AAPS PharmSciTech. 2007; 8(1): Article 15.
- Ali J, Arora S, Ahuja A, Babbar AK, et al. Formulation and development of hydrodynamically balanced system for metformin: In vitro and in vivo evaluation. Eur J Pharm Biopharm. 2007; 67: 196–201.
- Wamorkar VV, Varmal MM, Vijay Kumar B, et al. Effect of hydrophilic and hydrophobic polymers and in vitro evaluation of hydrodynamically balanced system of metoclopramide hydrochloride. Int J Pharm Sci Nanotechnol. 2010; 3(3): 1129-35.
- Salunke RJ, Shahi SR and Atram SC. Formulation and evaluation of hydrodynamically balanced system of ciprofloxacin HCl. Int J Pharm Res Dev. 2009; 7: Article 001
- Popa N, Novac O, Profire L, et al. Inclusion and release of theophylline from chitosan based microparticles. Turk J Chem. 2010; 34: 255-62.
- Bhise SB and Aloorkar NH. Formulation and in vitro evaluation of floating capsules of theophylline. Indian J Pharm Sci. 2008; 70(2): 224-27.
- Varelas CG, Dixon DG and Steiner C. Zero-order release from biphasic polymer hydrogels. J Control Release. 1995; 34: 185-92.
- Mulye NV and Turco ST. An examination of assumptions underlying the firstorder kinetic model for release of water soluble drugs from dicalcium phosphate dehydrate matrices. Drug Dev Ind Pharm. 1996; 22: 673-679.
- Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961; 50: 874–75.
- Korsmeyer RW, Gurny R, Docler E, et al. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15: 25-35.
- Sriamornsak P and Sungthongjeen S. Modification of theophylline release with alginate gel formed in hard capsules. AAPS PharmSciTech. 2007; 8: Article 51.
- Veski P and Marvola M. Sodium alginate as diluents in hard gelatine capsules containing ibuprofen as a model drug. Pharmazie. 1993; 48: 757-60.
- Veski P, Marvola M, Smal J, et al. Biopharmaceutical evaluation of pseudoephedrine hydrochloride capsules containing different grades of sodium alginate. Int J Pharm. 1994; 111: 171-79.
- Ojantakanen S, Hannula A-M, Marvola M, et al. Bioavailability of ibuprofen from hard gelatin capsules containing sucralfate or sodium alginate. Eur J Pharm Biopharm. 1993; 39: 197-201.
- Nayak AK, and Malakar J. Formulation and in vitro evaluation of gastroretentive hydrodynamically balanced system for ciprofloxacin HCl. J Pharmacy Educ Res. 2010; 1(2): 65-68.
- Siepmann J and Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Del Rev. 2001; 48: 139–57.
- US Patent No. 2003/0104053 A1. Gusler G, Berner B, Chau M, et al. Optimal polymer mixtures for gastric retentive tablets; 2001.
- Tiwari SB and Rajabi-Siahboomi AR. Applications of complementary polymers in HPMC hydrophilic extended release matrices. Drug Deliv Technol. 2009; 9(7): 20-27.
- Li H, Hardy RJ, and Gu X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech. 2008; 9: 437-43.