Formulation and evaluation of enteric coated tablets of proton pump inhibitor
- *Corresponding Author:
- Anroop B Nair
M.M. College of Pharmacy, M.M. University, Mullana, Ambala, India
E-mail: banroop@gmail.com
Date of Received: 12-05-2010
Date of Modified: 06-08-2010
Date of Accepted: 18-09-2010
Available Online: 15-11-2010
Abstract
The present study was an attempt to formulate and evaluate enteric coated tablets for esomeprazole magnesium trihydrate. Different core tablets were prepared and formulation (F-1) was selected for further enteric coating, based on the disintegra-tion time. Seal coating was applied to achieve 3% weight gain using opadry®. Enteric coating was carried out using different polymers like Eudragit L-30 D-55, hydroxy pro-pyl methylcellulose phthalate, cellulose acetate phthalate and Acryl-EZE® to achieve 5% weight gain. Disintegration studies showed that the formulations failed in 0.1 N HCl media. Hence the quantity of enteric coating was increased to 8% w/w. In vitro analysis of the developed tablets was carried out. Results from disintegration time and dissolution rate studies indicate that all the esomeprazole enteric tablets prepared pos-sess good integrity, desirable for enteric coated tablets. Among the polymers studied, the methacrylic polymers exhibited better dissolution rate than the cellulose polymers. Stability studies indicate that the prepared formulations were stable for a period of three months. This study concluded that enteric coated tablets of esomeprazole can be prepared using any of the enteric coating polymer studied using a minimal weight gain of 8%.
Keywords
Esomeprazole, enteric coating, tablets, Eudragit, HPMCP, CAP, stability.
Abbreviations
CAP – Cellulose acetate phthalate
FDA – Food drug administration
HCl – Hydrochloric acid
HPLC – High performance liquid chromatography
HPMCP – Hydroxy propyl methyl cellulose phthalate
IR – Infra red
PVP – Polyvinyl pyrrolidone
RH – Relative humidity
USP – United states Pharmacopoeia
Introduction
Oral site-specific drug delivery systems have attracted a great deal of interest recently for the local treatment of a variety of bowel diseases and also for improving systemic absorption of drugs, which are unstable in the stomach. However, the micro environment in the gastrointestinal tract and varying absorption mechanisms generally causes hindrance for the formulation scientist in the development and optimization of oral drug delivery. Delivery of therapeutic agent into the intestinal region could be accomplished by the application of an enteric coating on a solid dosage form [1]. Several approaches have been attempted and reported during the last decade to develop new methodologies for site-specific drug release, including pH-sensitive drug release and time-controlled drug release. Among these, the time-controlled release systems such as sustained or delayed-release dosage forms are very promising [1]. Nevertheless, due to the potentially large variation of gastric emptying time of dosage forms in humans, these dosage form may show high inter patient variability in the site of drug delivery. On the other hand, pH-sensitive delivery systems such as enteric-coated dosage forms offer a simple and practical means for intestinal drug delivery [1].
Esomeprazole magnesium trihydrate, is a classical example of proton pump inhibitors and is approved by FDA for the treatment of symptomatic gastroesophageal reflux disease, short-term treatment and maintenance of erosive esophagitis. Esomeprazole is an S-isomer of omeprazole and the first proton pump inhibitor to be developed as an optical isomer [2]. The drug has an improved pharmacokinetic profile, resulting in increased systemic exposure and less inter individual variability compared with omeprazole, and more effective suppression of gastric acid production compared with other proton pump inhibitors. Its bioavailability is 89% and plasma elimination half life is 1.5 h [3]. The stability of esomeprazole magnesium trihydrate decreases with a corresponding decrease in the pH of the media. Hence, the exposure of esomeprazole magnesium trihydrate to the acidic contents of the stomach would lead to significant degradation of the drug and would result in reduced bioavailability [2]. Few attempts have been made to deliver this drug by peroral route in the form of enteric coated granules, solid dispersion, suspension and matrix tablets [4-6].
A number of enteric coating polymers are available and capable of protecting the drug core from the aggressive environments of the stomach [7-10]. Being soluble at higher pH values, these polymers dissolve in the intestine and release the core for ready action. These polymers include several synthetic polymers like polymethacrylates (Eudragits), cellulose acetate phthalate (CAP), hydroxy propyl methyl cellulose phthalate (HPMCP). The aim of the present study was to compare the suitability of these renowned polymers to develop enteric coated tablets of a very sensitive proton pump inhibitor, esmoperazole.
Materials and Methods
Materials
Esomeprazole magnesium trihydrate, Eudragit L-30 D-55, hydroxy propyl methyl cellulose phthalate and cellulose acetate phthalate (Torrent Pharmaceutical Ltd., Baddi, India), acryl-EZE® and opadry® (Colorcon, Goa, India) and other additives were procured commercially. All the reagents and solvents used were of analytical grade
In vitro analysis of the prepared tablets was carried out as per the requirements of enteric coated tablets as specified in official pharmacopoeia [11].
Drug excipient interaction study
Active drug blended with individual excipients were taken in 1:1 ratio, filled in closed vials and placed in stability chambers at 35°C ± 2°C / 60% ± 5% RH for a period of 4 weeks. Samples were analyzed by IR.
Preparation of core tablets
Dummy granules for tablets were prepared by wet granulation method. The respective ingredients (polymer, and additives) were passed through a sieve no. 60 (250 μm) and blended with a turbula mixer (Analytical Technology, Bangalore, India). Activation of PVP K-30 was done using isopropyl alcohol and the prepared granules were dried. The dried granules were mixed with drug and compressed on a 10-station tablet machine (Cadmach, Ahmedabad, India) using 7 mm biconvex round shaped die and punches. Th ree batches were prepared for each formulation. The detailed compositions of esomeprazole core tablet formulations are given in Table 1.
| Ingredients (mg) | Formulations | ||||
|---|---|---|---|---|---|
| F1 (100/0)** | F2 (0/100)** | F3 (50/50)** | F4 (60/40)** | F5 (70/30)** | |
| Esomeprazole Magnesium Trihydrate* | 44.5 | 44.5 | 44.5 | 44.5 | 44.5 |
| Mannitol | 59.0 | - | 29.5 | 35.4 | 41.3 |
| Dibasic calcium phosphate | - | 59.0 | 29.5 | 23.6 | 17.7 |
| Sodium bicarbonate | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Poly vinyl pyrrolidone | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| (K-30) | |||||
| Croscarmellose sodium | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Magnesium stearate | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Talc | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Isopropyl alcohol | QS | QS | QS | QS | QS |
| Total weight | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
*Esomeprazole magnesium trihydrate 44.5mg is equivalent to 40mg of esomeprazole.
** (a/b) = a indicates mannitol and b indicates dibasic calcium phosphate in percentage
Table 1: Composition of various core tablet formulations
Preparation of enteric coated tablets
Seal coat of opadry® was applied to the tablets up to a weigh gain of 3%. Then the seal coated tablets were enteric coated with different enteric coating material such as Eudragit L-30 D-55, hydroxy propyl methyl cellulose phthalate, cellulose acetate phthalate and acryl-EZE®. The detailed compositions of esomeprazole enteric coated tablet formulations are given in Table 2
| Ingredients (mg) | Formulations | |||||||
|---|---|---|---|---|---|---|---|---|
| F1a* | F1b* | F1c* | F1d* | F1e** | F1f** | F1g** | F1h** | |
| Eudragit L-30 D-55 | 7.6 | - | - | - | 12.17 | - | - | - |
| HPMC-P | - | 7.6 | - | - | - | 12.17 | - | - |
| CAP | - | - | 7.6 | - | - | - | 12.17 | - |
| Acryl-EZE® | - | - | - | 7.6 | - | - | - | 12.17 |
| Triethyl citrate (5%) | 0.74 | 0.74 | 0.74 | 0.74 | 1.18 | 1.18 | 1.18 | 1.18 |
| Talc (10%) | 0.93 | 0.93 | 0.93 | 0.93 | 1.48 | 1.48 | 1.48 | 1.48 |
| Isopropyl alcohol | - | q.s. | q.s. | - | - | q.s. | q.s. | - |
| Di chloro methane | - | q.s. | q.s. | - | - | q.s. | q.s. | - |
| Purified water | q.s. | - | - | q.s. | q.s. | - | - | q.s. |
| Total weight | 194.67 | 194.67 | 194.67 | 194.67 | 200.23 | 200.23 | 200.23 | 200.23 |
*5% weight gain in 1st four formulations i.e. F1a to F1d.
**8% weight gain in the remaining four formulations i.e. F1e to F1h.
Table 2: Composition of enteric coating (5% and 8% weight gain)
Evaluation of Granules
Bulk density, tapped density and Carr’s index
Ten grams of granules were introduced into a clean, dry 100 ml measuring cylinder and the volume was recorded. The cylinder was then tapped 25 times from a constant height and the tapped volume was read. The bulk density and tapped density were calculated as the ratio of the granules mass and the respective volumes. Carr’s index (I) was calculated using the equation [3]:
I = Dt – Db / Dt x 100
where Dt is the tapped density of the powder and Db is the bulk density of the powder.
Angle of repose
The fixed funnel method was employed for determining the angle of repose. The granules were poured carefully until the apex of the conical pile just touches the tip of the stem of the funnel. The angle of repose was calculated using the equation [12]:
Tan α = H/R
where H is the height of the pile and R is the radius of the base of the conical pile.
Evaluation of Tablets
Hardness
The tablet crushing strength was tested by commonly used Monsanto type tablet hardness tester (IEC, Mumbai, India). A tablet is placed between the anvils and the crushing strength, which causes the tablet to break, is recorded [12].
Friability Test
Tablet strength was tested by Roche friabilator (Electrolab, Bangalore, India). Pre weighed tablets were given 100 revolutions in 4 min and were dedusted. The percentage weight loss was calculated by reweighing the tablets [12].
Uniformity of weight
Randomly selected twenty tablets were weighed individually and together in a single pan balance (Shimadzu, AX200, Japan). The average weight was noted and standard deviation calculated [12].
Disintegration time
Disintegration time was determined using the disintegration apparatus USP (Electrolab, Bangalore, India) in 0.1N HCl for 2 h and then in phosphate buffer pH 6.8 maintaining the temperature at 37 ± 2°C [13].
Drug content studies
The drug content in tablets were determined by randomly choosing twenty tablets of each formulation and powdered using mortar & pestle. A quantity equivalent to 40 mg of esomeprazole magnesium trihydrate was weighed, dissolved in mobile phase and diluted suitably. The amount of drug was determined by injecting 20 μl of the sample in a HPLC system (Shimadzu, LC-10ATVP, Japan) consisting of a Phenomenex C18 analytical column (4.6 X 250 mm, Luna, 5.0 μm). The column was maintained at ambient temperature. The compounds were eluted at a flow rate of 1.0 ml/min using a mobile phase of Acetonitrile: Phosphate buffer pH 6.8 (60:40 v/v) [14]. The column effluent was monitored at 203 nm.
In vitro Dissolution tests
Drug release profile was evaluated in vitro using a dissolution test apparatus (Electro Lab, TDT-08L, Mumbai, India). The USP XIII Type II (paddle type) method was selected to perform the dissolution profile of esomeprazole magnesium trihydrate. The dissolution for all the formulations was carried out according to US Pharmacopoeia [13] for 2 h in 0.1N HCl and then media was changed into phosphate buffer pH 6.8. The temperature was maintained at 37 ± 0.5°C and a constant paddle rotation speed of 100 rpm. Samples (10 ml) were withdrawn at regular intervals and filtered through membrane filter (pore size 0.22 μm). The samples were analyzed by HPLC.
Stability studies
The stability studies were carried out at 25°C ± 2°C / 60% ± 5% RH, 35°C ± 2°C / 60% ± 5% RH and 40°C ± 2°C / 75% ± 5% RH for selected formulations for 3 months.
Statistical evaluation
The data were statistically analyzed by one-way analysis of variance (ANOVA) and student’s t-test.
Results and discussion
The compatibility studies to assess any possible interaction between the drug and excipients were carried out and analyzed using IR for a period of 4 weeks. The observed IR spectra did not show any alteration in IR peaks, suggesting no possible interaction between excipients and esomeprazole (Data not shown).
As mentioned above, exposure of esomeprazole to acidic environment leads to significant degradation of the drug and reduces bioavailability as well. Enteric-coated tablets with a low core pH will have longer in vivo disintegration time, due to the suppression of ionization of enteric coating polymers in the acidic environment. As a result, tablets with a high proportion of an acidic therapeutic agent or acidic excipients would probably exhibit retardation in dissolution if directly enteric coated. Considering the dissolution in general and stability in particular, the pH of the core tablet was basified using sodium bicarbonate (50 mg). Five different core tablets (F1- F5) of esomeprazole (40 mg) were prepared with varying concentration of diluents (dibasic calcium phosphate and mannitol) (Table-1). Excipients such as mannitol or dibasic calcium phosphate (diluents), sodium bicarbonate (stabilizer), polyvinyl pyrrolidone (binder), croscarmellose sodium (disintegrant), magnesium stearate (lubricant) and talc (glidant) were used to prepare the core tablets. The prepared core tablets were subjected to disintegration test at pH 6.8. Based on the disintegration test, F1 formulation was selected for further enteric coating as this had shown minimum disintegration time (1.5 ± 0.30 min). The granules used for preparing the F-1 formulations exhibited ideal properties for tablet compression (bulk density; 0.47 ± 0.05 g/cc), tapped density (0.58 ± 0.03 g/cc), angle of repose (29.65 ± 1.25 θ), Carr’s Index (18.96 ± 0.89). Moreover, the physical properties of compressed tablets (F-1) exhibited good physical integrity (hardness: 5.6 ± 0.42 kg/cm2), (friability: 0.27 ± 0.06%), (weight variation: 181.25 ± 5.83). Further, the core tablets (F-1) were seal coated with a commonly used polymer, Opadry® up to 3% weight gain.
The purpose of enteric-coating is to delay the release of drugs that are inactivated by the stomach contents or may cause nausea or irritation of gastric mucosa. The design of an enteric coating may be based on the transit time required for passage to the intestine and may be accomplished through coatings of sufficient thickness. Enteric-coating materials may be applied to either whole compressed tablets or to drug particles or granules used in the fabrication of tablets or capsules. Several commercially available polymers are employed in enteric coating the tablets. In the current study we have selected four enteric coating materials i.e., Eudragit L-30 D-55, hydroxy propyl methylcellulose phthalate, cellulose acetate phthalate and Acryl-EZE®. In general, these substances are anionic polymers or copolymers which are insoluble in acidic media but acquire water solubility at near neutral pH values due to ionization of functional groups along the polymer chain. In most applications, Eudragit L30 D55 dispersions require the addition of a suitable plasticizer and detackifier such as triethyl citrate and talc, respectively, for proper film forming properties and ease of processability [15]. Hence triethyl citrate and talc were incorporated in the formulations. Four formulations (F1a-F1d) were enteric coated to achieve 5% weight gain using the different enteric coating materials selected. The characteristics of the enteric coated tablets are presented in Table 3. The weight variation and the drug content of all the formulations were found to be with-in the official limit. However, the formulations failed the disintegration test in 0.1 N HCl (Table-3). Hence, four more formulations were prepared (F1e-F1h) with greater amount of enteric coating material (weight gain of 8%). It is evident from Table 3 that the increase in the enteric coating weight (up to 8%) resulted in non disintegration of the tablets in the acid media (0.1 N HCl) during the study period (2 h). The observed data is in agreement with the reported data showing the potential of these polymers to prevent disintegration in acidic media [16,17]. However, the disintegration test indicated significant difference (P< 0.001) in the disintegration time of the formulations (F1a-F1h) when evaluated in phosphate buffer pH 6.8. The data also suggests that the disintegration time is independent of the enteric coating weight gain but most likely depends on the polymer, in the current experimental condition (Table-3).
| Parameters | Formulations | |||||||
|---|---|---|---|---|---|---|---|---|
| F1a | F1b | F1c | F1d | F1e | F1f | F1g | F1h | |
| Weight variation | 193.63 ± 4.69 | 195.24 ± 4.73 | 195.52 ± 4.58 | 194.09 ± 4.52 | 202.06 ± 5.16 | 198.37 ± 5.09 | 200.41 ± 5.14 | 201.62 ± 5.11 |
| Thickness (mm) | 3.56 ± 0.08 | 3.57 ± 0.07 | 3.55 ± 0.09 | 3.57 ± 0.08 | 3.58 ± 0.06 | 3.57 ± 0.09 | 3.56 ± 0.08 | 3.59 ± 0.06 |
| Drug content (%) | 92.26 ± 2.67 | 94.84 ± 2.75 | 91.68 ± 2.69 | 93.12 ± 2.72 | 98.73 ± 2.65 | 95.09 ± 2.68 | 92.65 ± 2.71 | 98.56 ± 2.65 |
| Disintegration (0.1N HCl) (min) | 115.5 ± 7.5 | 86.0 ± 11.6 | 92.6 ± 14.5 | 110.27 ± 5.9 | - | - | - | - |
| Disintegration (pH 6.8) (min) | 11.35 ± 0.33 | 9.45 ± 0.35 | 10.05 ± 0.37 | 10.55 ± 0.29 | 8.50 ± 0.34 | 12.25 ± 0.28 | 12.40 ± 0.36 | 10.05 ± 0.33 |
*Each value is the mean ± SE (n=6)
Table 3: Characteristics* of esomeprazole enteric-coated tablets
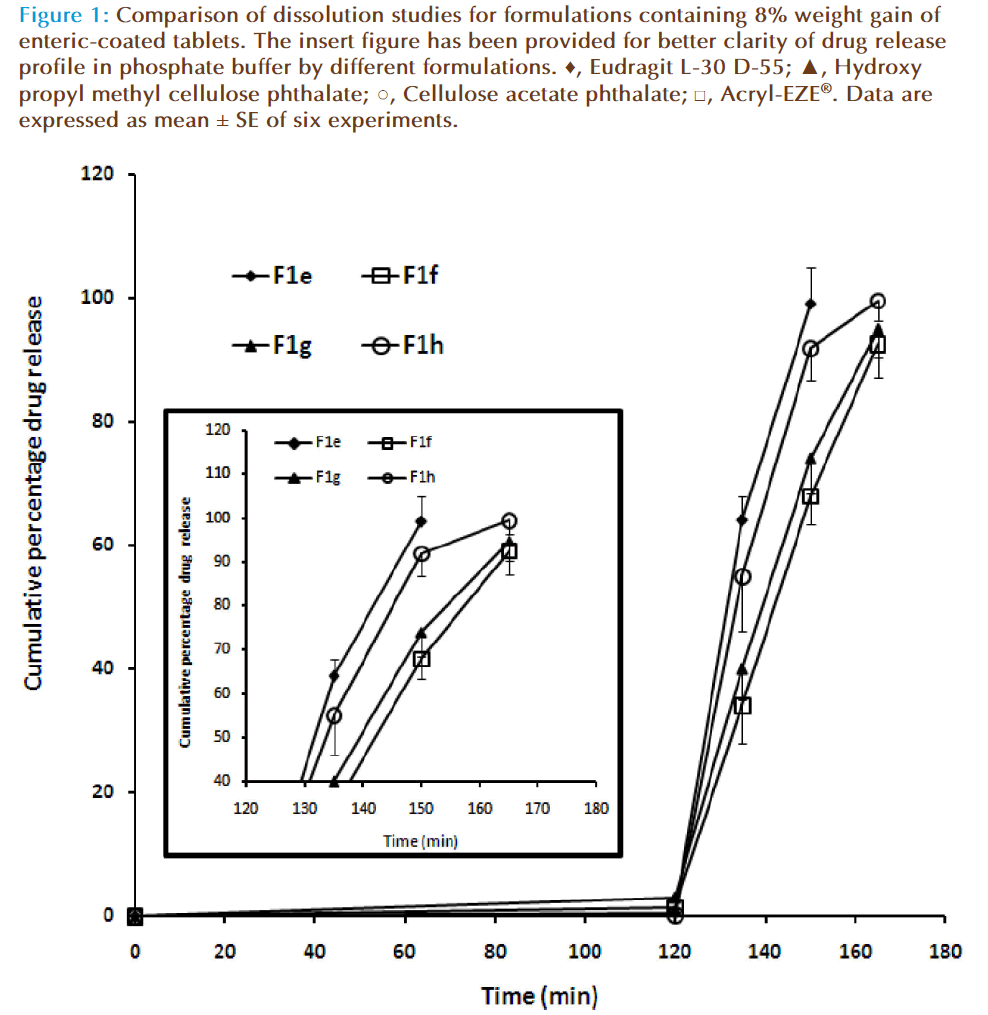
Dissolution analysis was employed to assess the effect of the enteric coat composition and coverage levels on the release of the formulations. In vitro drug release was carried out for formulations with 8% weight gain of enteric coated tablets (F1d-F1h) in 0.1 N HCl for 2 h followed by phosphate buffer (pH 6.8) for 45 mins. Figure 1 compares the dissolution profile of enteric coated esomeprazole tablets prepared using different coating materials (8% weight gain) in different buffers. It is evident from the figure that all the four formulations demonstrated excellent physical resistance to the acid medium with the acid uptake value between 0.27-2.87% in 2 h. Altering the media to basic (phosphate buffer-pH 6.8) leads to rapid release of the esomeprazole from all the formulations evaluated. Th is observation is anticipated, as these enteric coated polymers have shown the similar effect in earlier reports [18-20]. The t50 values for all the formulations varied between 125 to 145 min, while the t90 values were found to be between 145 -165 min. The cumulative percentage released at the end of the study was found to be relatively high with formulations made by enteric coating with methacrylic acid co-polymers [Eudragit L-30 D-55 (F1e: 99.12 ± 4.31%) and Acryl-EZE® (F1h: 99.58 ± 3.28%)] than the tablets coated with HPMCP (F1f: 92.64 ± 5.48%) and CAP (F1g: 94.89 ± 4.51%).
Figure 1: Comparison of dissolution studies for formulations containing 8% weight gain of enteric-coated tablets. The insert figure has been provided for better clarity of drug release profile in phosphate buffer by different formulations. ♦, Eudragit L-30 D-55; ▲, Hydroxy propyl methyl cellulose phthalate; ○, Cellulose acetate phthalate; □, Acryl-EZE® Data are expressed as mean ± SE of six experiments.
The stability studies were performed for the four formulations (F1e-F1h) at 25°C ± 2°C / 60% ± 5% RH, 35°C ± 2°C / 60% ± 5% RH and 40°C ± 2°C / 75% ± 5% RH. The samples were analyzed for disintegration time, drug content and drug release. The results indicated that the all the four formulations were stable, and did not show any significant difference in drug content, disintegration time and dissolution rate after a study period of three months.
Conclusion
Esomeprazole core tablets were prepared and stabilized using sodium bicarbonate as a stabilizer. A seal coat of 3% weight gain using opadry® was sufficient to protect the tablets from the acid coat of the enteric layer. Enteric coating was done using four different enteric coating materials (Eudragit L-30 D-55, hydroxy propyl methylcellulose phthalate, cellulose acetate phthalate, Acryl-EZE®) to achieve 5% weight gain. Evaluation of these tablets indicated that the tablets coated with HPMCP and CAP failed the disintegration test in 0.1 N HCl. However, formulations which were enteric coated to 8% weight gain could pass the disintegration test carried out at pH 1.2. The study indicates that out of the four polymers studied, methacrylic acid polymers are most suitable for enteric coating. These provide greater protection to the core under acidic condition while at the same time show the fastest drug release under intestinal pH. The above formulations were found to be stable for three months.
Conflict of Interest
There are no conflicts of interest.
References
- Fukui E, Miyamura N, Uemura K, et al. Preparation of enteric-coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm. 2000; 204: 7-15.
- Johnson DA. Review of esomeprazole in the treatment of acid disorders. Expert Opin Pharmacotherapy. 2003; 4: 253-264.
- Brunton LL, Lazo JS, Parker KL, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, New York, McGraw Hill, 2006.
- Biswas BK, Islam, S, Begum F,et al. In vitro release kinetic study of esomeprazole magnesium from methocel K15M and methocel K100 LVCR matrix tablets. Dhaka Univ J Pharm Sci. 2008: 7: 39-45.
- Bladh N, Blychert E, Johansson K, et al. A new esomeprazole packet (sachet) formulation for suspension: in vitro characteristics and comparative pharmacokinetics versus intact capsules/tablets in healthy volunteers. Clin Ther. 2007; 4: 640-649.
- Xie Y, Xie P, Song X, et al. Preparation of esomeprazole zinc solid dispersion and study on its pharmacokinetics. Int J.Pharm. 2008; 360: 53-57.
- Murthy KS, Kubert DA, Fawzi MB. In vitro release characteristics of hard shell capsule products coated with aqueous- and organic-based enteric polymers. J Biomater Appl. 1988; 3: 52-79.
- Sinha VR, Kumria R. Coating polymers for colon specific drug delivery: A comparative in vitro evaluation. Acta pharm. 2003; 53: 41-47.
- Marvola M, Nykänen P, Rautio S, et al. Enteric polymers as binders and coating materials in multiple-unit site-specific drug delivery systems. Eur J Pharm Sci. 1999; 7: 259-267.
- Nesbitt RU, Goodhart FW, Gordon RH. Evaluation of polyvinyl acetate phthalate as an enteric coating material. Int J Pharm. 1985; 26: 215-226.
- British Pharmacopoeia, British Pharmacopoeia Commission, London, 2009.
- Keith M. Compression and consolidation of the powdered solids. In: Leon Lachman, Liberman HA, Kanig JL. (eds). Theory and practice of Industrial Pharmacy. ed 3, Mumbai, India: Varghese publishing house, 1991, 66-72.
- USP XXIV, 17Thed., Rockville, 1947, MD 2000.
- Onal A, Oztunc A. Development and validation of high performance liquid chromatographic method for the determination of esomeprazole in tablets. J Food Drug Analysis. 2006; 14: 12-18.
- Crotts G, Sheth A, Twist J et al. Development of an enteric coating formulation and process for tablets primarily composed of a highly water-soluble, organic acid. Eur J Pharm. Biopharm. 2001; 51: 71-76.
- Felton LA, Haase MM, Shah NH, et al. Physical and enteric properties of soft gelatin capsules coated wiTheudragit® L 30 D-55. 1995; 113: 17-24.
- El-Malah Y, Nazzal S. Novel use of Eudragit NE 30D/Eudragit L 30D- 55 blends as functional coating materials in time-delayed drug release applications. Int J Pharm. 2008; 357: 219-227.
- Sauer D, Watts AB, Coots LB, et al. Influence of polymeric subcoats on the drug release properties of tablets powder-coated with pre-plasticized Eudragit® L 100-55. Int J Pharm. 2009; 367: 20-28.
- Wei G, Knoch A, Laicher A, et al. Simple coacervation of hydroxypropyl methylcellulose phthalate (HPMCP) II. Microencapsulation of ibuprofen. Int J Pharm. 1995; 124: 97-105.
- Kim H, Park JH, Cheong W et al. Swelling and drug release behavior of tablets coated with aqueous hydroxypropyl methylcellulose phthalate (HPMCP) nanoparticles. J Control Release 2003; 89: 225-233.