Formulation and evaluation of controlled porosity osmotic pump for oral delivery of ketorolac
- *Corresponding Author:
- Dr. Fatima Sanjeri Dasankoppa
Assistant Professor, Department of Pharmaceutics, K.L.E.U’s College of Pharmacy, Vidyanagar, Hubli - 580 031, Karnataka, India.
E-mail: sanjeri@yahoo.co.uk
Abstract
Background: The osmotic drug delivery systems suitable for oral administration typically consist of a compressed tablet core that is coated with a semipermeable membrane that has an oriô€ÂÂÂÂÂÂÂÂice drilled on it by means of a laser beam or mechanical drill. Ketorolac is a nonsteroidal agent with powerful analgesic. Oral bioavailability of ketorolac was reported to be 90% with very low hepatic ô€ÂÂÂÂÂÂÂÂirst-pass elimination; the biological half-life of 4-6 hours requires frequent administration to maintain the therapeutic effect. Aim: The aim of the current study was to design a controlled porosity osmotic pump (CPOP)based drug delivery system for controlled release of an NSAID agent, ketorolac tromethamine, which is expected to improve patient compliance due to reduced frequency; it also eliminates the need for complicated and expensive laser drilling and maintain continuous therapeutic concentration. Design: The CPOP was designed containing pore-forming water-soluble additives in the coating membrane, which after coming in contact with water, dissolve, resulting in an in situ formation of a micro porous structure. Materials and Methods: The effect of different formulation variables, namely level of pore former (PVP), plasticizer (dibutyl phthalate) in the membrane, and membrane weight gain were studied. Results and Conclusion: Drug release was inversely proportional to the membrane weight but directly related to the initial concentration of pore former (PVP) in the membrane. Drug release was independent of pH and agitational intensity, but dependent on the osmotic pressure of the release media. Based on the in vitro dissolution proô€ÂÂÂÂÂÂÂÂile, formulation F3C1 (containing 0.5 g PVP and 1 g dibutyl phthalate in coating membrane) exhibited Peppas kinetic with Fickian diffusion-controlled release mechanism with a drug release of 93.67% in 12 hours and hence it was selected as optimized formulation. SEM studies showed the formation of pores in the membrane. The formulations were stable after 3 months of accelerated stability studies. CPOP was designed for effective administration of drugs for prolonged period of time.
Keywords
Controlled porosity osmotic pump, ketorolac tromethamine, pore former, peppas kinetics, scanning electron microscopy, stability study
Introduction
In recent years, considerable attention has been focused on the development of novel drug delivery systems (NDDS).[1] Conventional drug delivery systems have no control over the drug release and effective concentration at the target site. This kind of dosing pattern may result in constantly changing, unpredictable plasma concentrations; hence oncedaily controlled release preparation is often desirable. Drug release from oral controlled release dosage forms may be affected by pH, gastrointestinal motility, and presence of food in the gastrointestinal tract.[2] One practical approach with a potential to overcome the abovesaid disadvantages is the osmotic drug delivery system, [3,4] wherein drugs can be delivered in a controlled pattern over a long period of time by the process of osmosis.
The osmotic drug delivery systems suitable for oral administration typically consist of a compressed tablet core that is coated with a semipermeable membrane that has an orifice drilled on it by means of a laser beam or mechanical drill.[5]
To obviate the need for complicated laser drilling, tablets coated with a membrane of controlled porosity have been described. These membranes consist of a leachable material which dissolves upon contact with water, leaving behind the pores through which the drug solution is pumped out. However, due to the relatively low permeability of the dense coatings, osmotic delivery of drugs with moderate to low solubility is limited.[6,7]
Ketorolac is a nonsteroidal agent with powerful analgesic and low antiinflammatory activity, widely used in the management of both moderate and severe pain. Although oral bioavailability of ketorolac was reported to be 90% with very low hepatic first-pass elimination, the biological half-life of 4–6 hours requires frequent administration to maintain the therapeutic effect. The long-term use of currently available dosage forms of ketorolac may result in gastrointestinal ulceration and acute renal failure.[8]
In the present investigation, an attempt will be made to design a simplified controlled porosity osmotic system of ketorolac and development of sustained release tablet dosage, which is expected to improve patient compliance due to reduced frequency; [9] it also eliminates the need for mplicated and expensive laser drilling and maintain continuous therapeutic concentration.
Materials and Methods
Materials
Ketorolac tromethamine (KT) was provided as a gift sample from MSN Laboratories Ltd., Hyderabad, Symed Pharma Ltd., Hyderabad; dextrose monohydrate and microcrystalline cellulose as a gift sample from Micro labs Pvt. Ltd., Bangalore; polyvinylpyrollidone (PVP), talc, and magnesium stearate as a gift sample from Elegant Drugs Pvt. Ltd., Hubli; and Lake Tartrazine as a gift sample from Standardcon Pvt. Ltd., Mumbai. Following chemicals and excipients were purchased from commercial sources and used as such: ethyl cellulose (Thomas baker chemicals Pvt. Ltd., Mumbai), Dibutyl phthalate (DBP) (Himedia lab. Pvt. Ltd., Mumbai).
Study of interexcipients and drug compatibility study
A compatibility study was performed for ketorolac tromethamine and in combination with different polymer and excipients in the ratio of 1:1. The sample was exposed to 40°C/75% RH (stability chamber, TH 50S Thermolab, Mumbai) for 3 months and was analyzed by differential scanning calorimetric (DSC).[3]
Formulation of ketorolac tromethamine core tablets
Core tablets of ketorolac tromethamine were prepared by direct compression, [10] and the batch size was kept as 50 tablets. Required amounts of ketorolac tromethamine, dextrose monohydrate, microcrystalline cellulose were weighed and transferred into mortar pastel. The dry powders were blended for 5 minutes. The mixture was passed through 44-mesh sieve. The blend was dried at 45°C for 20 minutes, then blended with magnesium stearate and talc (all 60-mesh passed) and compressed into tablets having an average weight of 100 mg using a multistation tablet-punching machine (Rimek mini press I) fitted with 9.5 mm round standard concave punches. The formula of core formulation of ketorolac tromethamine is listed in Table 1.
| Ingredient (s) | Formulation codes | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| Ketorolac tromethamine | 20 | 20 | 20 |
| Dextrose monohydrate | 10 | 20 | 30 |
| Microcrystalline cellulose | 67 | 57 | 47 |
| Magnesium steçarate | 1 | 1 | 1 |
| Talc | 2 | 2 | 2 |
| Total weight | 100 | 100 | 100 |
All quantities were taken in mg
Table 1: Formulation of ketorolac tromethamine core tablets.
Coating of core tablets
The composition of the coating solution used for coating of ketorolac tromethamine tablets is given in Table 2. Various components of the coating solution were added to the solvent mixture in a sequential manner. The component added first was allowed to dissolve before addition of the next component and mixed until a homogeneous mixture was formed.
| Ingredients | Coating solution codes | ||
|---|---|---|---|
| C1 | C2 | C3 | |
| Ethyl cellulose | 5 | 5 | 5 |
| Dibutyl phthalate # | 1 | 1 | 1 |
| PVP K30 | 10 | 20 | 30 |
| Lake tartrazine | 0.1 | 0.1 | 0.1 |
| Coat weight gain (CWG) | 5 | 5 | 5 |
All quantities were taken in %, Toluene:ethanol [8:2] ratio was used as a coating solvent
Table 2: Formula of coating solutions.
The core tablets were loaded into the pan and spray nozzle was adjusted to spray on the upper half of the tablet bed. The operating condition was maintained as follows: pan speed 12 rpm, spray rate 02 ml/min, air temperature 35–40°C, atomization air pressure 6–10 psig, and distance from the tablet bed to the spray gun 15–20 cm. The coating was performed in a conventional coating pan of 12 inch (internal) diameter rotated on its horizontal axis at 45° inclination with a pilot spray gun type 68-S and drier fitted to coating pan. The tablets were sprayed by solution in the reservoir of the spray gun. The coating process was carried out till tablets attained a desired weight gain.[11] Composition of the tablets along with codes is tabulated Table 3.
| Ingredients (%) | Formulation codes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1C1 | F1C2 | F1C3 | F2C1 | F2C2 | F2C3 | F3C1 | F3C2 | F3C3 | |
| Ketorolac tromethamine | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Dextrose monohydrate | 10 | 10 | 10 | 20 | 20 | 20 | 30 | 30 | 30 |
| Microcrystalline cellulose | 67 | 57 | 47 | 67 | 57 | 47 | 67 | 57 | 47 |
| Magnesium stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Talc | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total weight of core tablet | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ethyl cellulose | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dibutyl phthalate # | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| PVP K30 | 10 | 20 | 30 | 10 | 20 | 30 | 10 | 20 | 30 |
| Lake tartrazine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Coat weight gain (CWG) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
Except (#) in g, Toluene:ethanol [8:2] ratio was used as a coating solvent .
Table 3: Composition of ketorolac tromethamine tablets
Content uniformity test
Ten tablets were finely powdered; quantity of the powder equivalent to 100 mg of ketorolac tromethamine was accurately weighed and diluted with distilled water to make concentration of 10 mcg/ml and measure the absorbance at 323 nm.[2,12]
Dimensions
Six tablets randomly picked from formulations were subjected for individual thickness and diameter measurements using dial-caliper (Mitutoyo, Japan).[11]
In vitro drug release
In vitro drug release of the formulations was carried out in a USP dissolution apparatus (paddle type) set at a rotating speed of 100 rpm and temperature of 37 ± 0.5°C. The dissolution medium (900 ml) was simulated gastric fluid (SGF IP 2007, pH 1.2) for the first 2 hours and simulated intestinal fluid (SIF IP 2007, pH 6.8) thereafter. Samples (5 ml) were withdrawn at 1 hour time intervals over a 12-hour period and the medium was replenished with fresh dissolution fluid. The samples were suitably diluted, analyzed spectrophotometrically at 323 nm, and drug release was computed.[4]
Measurement of the fi lm thickness
Following the completion of dissolution, the film was isolated from the tablets and dried at 40°C for 1 hour. The thickness was measured at three different points on the film using dialcaliper (Mitutoyo, Japan) and the mean values were taken.[4]
Mechanism of drug release
The drug release mechanism from coated tablets was studied by the following tests:
Effect of % coat weight gain
The different % coat weight gain in the coating formulation was verified and its effect on the drug release of optimized formulation was evaluated.[3]
Effect of pH
In order to study the effect of pH and to assure a reliable performance of the developed formulations independent of pH, release studies of the optimized formulations were carried out at pH 1.2 in simulated gastric fluid (SGF) and pH 6.8 in simulated intestinal fluid (SIF) and distilled water.[3]
Effect of agitational intensity
To study the effect of agitation intensity (rpm) of the dissolution medium, the release study was carried out using USP-type II dissolution apparatus (paddle type) at rotational speeds of 50, 100, and 150 using the dissolution medium (900 ml) of SGF of pH 1.2 for the first 2 hours and SIF of pH 6.8 thereafter.[3]
Effect of osmotic pressure
Optimized formulation were subjected to release studies in dissolution media containing dextrose monohydrate (osmotically effective solute) of varying strengths, to confirm the release mechanism by osmosis. Release studies were performed in 900 ml of osmotically active medium using USP-II dissolution apparatus at 100 rpm.[13]
Scanning electron microscopy (SEM) study
In order to elucidate the mechanism of drug release, optimized A2 coating membranes of tablets were subjected to scanning electron microscopy (SEM) studies before and after dissolution studies.[14,15]
Accelerated stability study
Optimized formulation (F3C1) was sealed in aluminum packaging coated inside with polyethylene. The packed tablets were placed in stability chambers maintained at 40 + 2°C and 75 + 5 % RH for 3 months in a stability chamber. The samples were withdrawn after 3 months and were evaluated for drug content and for in vitro drug release.[11]
Results and Discussion
Differential scanning calorimetric study
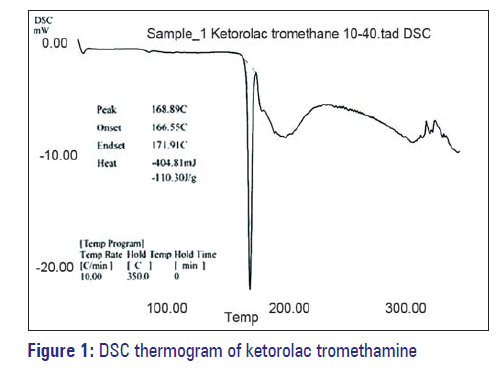
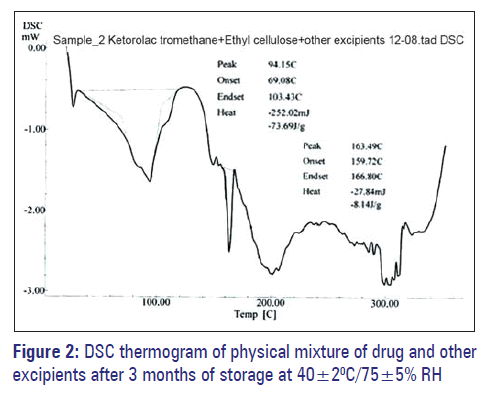
Figure 1 indicates that the melting of drug is at 168.89°C and is concordant with the literature value. In Figure 2, two endotherms were observed at 163.49°C and 94.15°C, endotherm at 163.49°C of drug and at 94.15°C of dextrose monohydrate and other excipients present in the physical mixture. Hence, ketorolac tromethamine was compatible with other excipients that are intended to be added into the formulation.
Core formulation of ketorolac tromethamine
The coated tablets were subjected to various physicochemical properties. All the batches exhibit good physicochemical properties and are to be used for further coating [Table 4].
| Formulation code | Weight variation | Hardness | Thickness | Friability | Drug | Film thickness |
|---|---|---|---|---|---|---|
| (mg ± S.D.) (n=20) |
(kg/cm2) (n=5) |
(mm ± S.D.) (n=10) |
(%) | content (%) |
(% ± S.D.) (n=3) |
|
| F1C1 | 100.40 ± 1.60 | 5.10 ± 0.10 | 3.218 ± 0.01 | 0.099 | 98.28 | 0.136 ± 0.01 |
| F1C2 | 100.70 ± 1.49 | 5.06 ± 0.08 | 3.219 ± 0.01 | 0.049 | 99.52 | 0.138 ± 0.01 |
| F1C3 | 100.45 ± 1.53 | 5.11 ± 0.10 | 3.212 ± 0.01 | 0.099 | 99.04 | 0.140 ± 0.005 |
| F2C1 | 100.50 ± 1.63 | 5.40 ± 0.07 | 3.213 ± 0.01 | 0.074 | 98.56 | 0.140 ± 0.005 |
| F2C2 | 100.45 ± 1.53 | 5.06 ± 0.08 | 3.218 ± 0.01 | 0.062 | 99.04 | 0.139 ± 0.015 |
| F2C3 | 100.55 ± 1.73 | 5.20 ± 0.27 | 3.218 ± 0.01 | 0.098 | 98.41 | 0.136 ± 0.005 |
| F3C1 | 100.40 ± 1.60 | 5.11 ± 0.10 | 3.212 ± 0.01 | 0.084 | 100.71 | 0.140 ± 0.010 |
| F3C2 | 100.30 ± 1.38 | 5.36 ± 0.15 | 3.216 ± 0.01 | 0.036 | 98.28 | 0.139 ± 0.015 |
| F3C3 | 101.20 ± 1.58 | 5.46 ± 0.11 | 3.215 ± 0.01 | 0.104 | 100.23 | 0.139 ± 0.015 |
Table 4: : The physicochemical properties of the controlled porosity osmotic pump.
Dissolution profi le of the CPOP
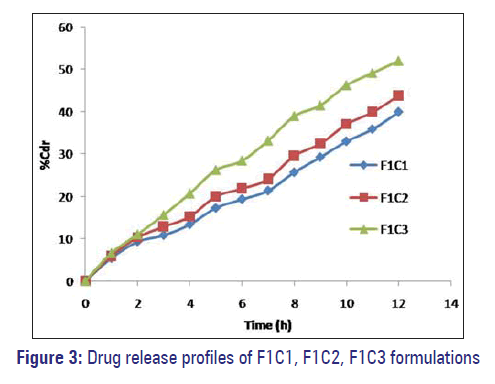
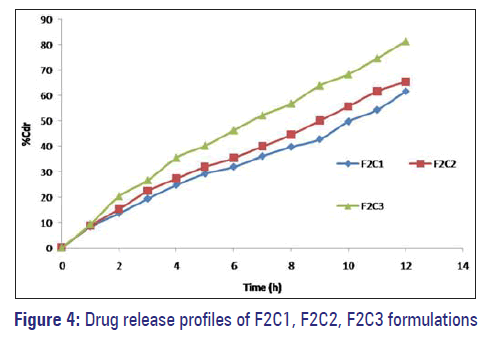
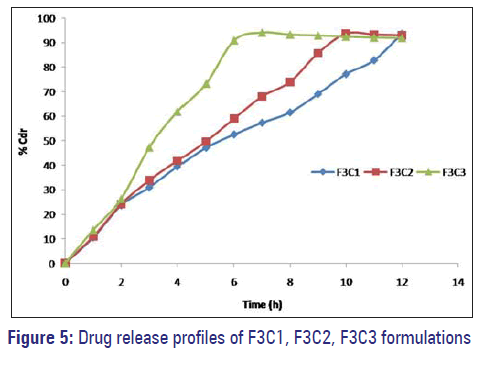
F1C1, F1C2, and F1C3 formulations exhibited 39.95%, 43.86%, and 52.02% drug release. F2C1, F2C2, and F2C3 formulations exhibited 61.54%, 65.28%, and 81.43% drug release at 12 hours respectively. F3C1, F3C2, and F3C3 formulations exhibited 93.67% drug release after 12 hours, 93.84% drug release after 10 hours and 94.18% drug release after 7 hours respectively [Figures 3–5].
Kinetics and mechanism of drug release
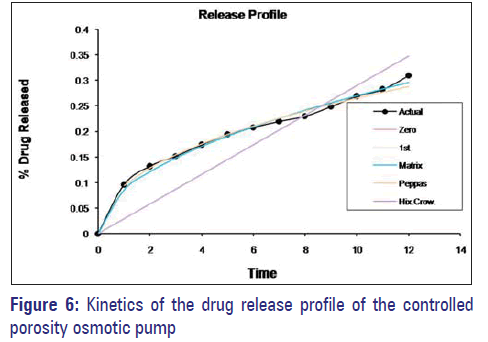
Dissolution data of the formulations were fitted to various mathematical models (zero-order, first-order, Peppas, and Hixon–Crowel) in order to describe the kinetics of drug release. The smallest value of the sum of squared residuals (SSR) and the highest value of the correlation coefficient (r) were taken as criteria for selecting the most appropriate model. It is clear that optimized formulas have n value 0.4499, indicating Fickian diffusion-controlled release [Figure 6 and Table 5].
| Model | Parameters used to asses the fit of model | |
|---|---|---|
| r | K | |
| Zero order | 0.8416 | 0.0290 |
| First order | 0.8422 | -0.0003 |
| Peppas | 0.9960 | 0.0942 |
| Hixon-Crowel | 0.8420 | -0.0001 |
| N | 0.4499 | |
r: correlation coefficient; k: release rate constant; SSR: sum of squared residuals; n: parameter for the Korsmeyer–Peppas equation
Table 5: Fitting of drug release data of the optimized formulation according to various mathematical models.
The aim of current study was to develop a 12-hour controlled release formulation. Based on the in vitro dissolution profile, formulation F3C1 shows the prominent % drug release, i.e., 93.67, in 12 hours and hence it was selected as optimized formulation.
Effect of different level of osmogent
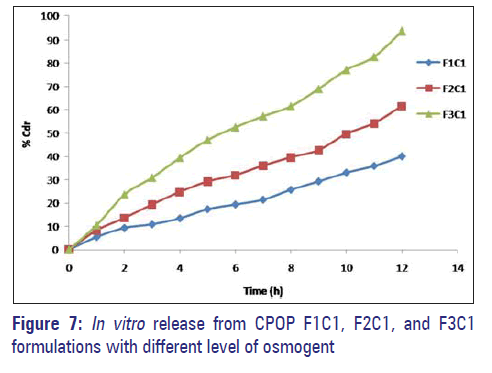
The amount of osmogent in the core formulation was varied and its effect on the drug release of the formulations was studied using osmogent, i.e., dextrose monohydrate, and the concentration was varied at three levels namely 10 mg, 20 mg, and 30 mg with coat weight gain of 5% [Figure 7].
Formulations F1C1, F2C1, and F3C1 containing 10 mg, 20 mg, and 30 mg of dextrose monohydrate exhibited 39.95%, 61.54%, and 93.67% drug release after 12 hours. Drug release is higher in the formulation F3C1 compared to F2C1 and F1C1 due to higher concentration of osmogent level, owing to greater osmotic pressure.
Effect of the different type and level of pore former
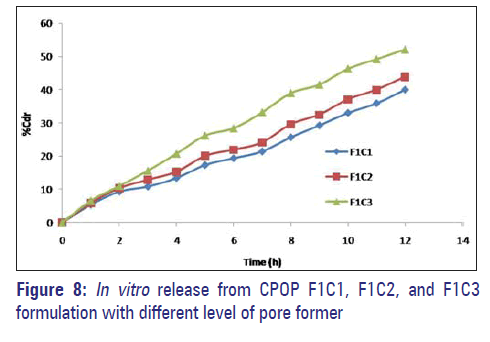
The amount of pore former in the coating formulation was varied and its effect on the drug release of the formulations was studied using a pore former, i.e., PVP, and the concentration was varied at three levels namely 0.5 g, 1 g, and 1.5 g with coat weight gain of 5%. The in vitro release profiles are shown in Figure 8.
Formulations F1C1, F1C2, and F1C3 containing 0.5 g, 01 g, and 1.5 g of PVP exhibited 39.95%, 43.86%, and 52.02% drug release respectively at the end of 12 hours. Drug release is higher in the formulation containing PVP 1.5 g; as the level of pore former increases, the membrane becomes more porous after coming in contact with the aqueous environment, resulting in faster drug release.
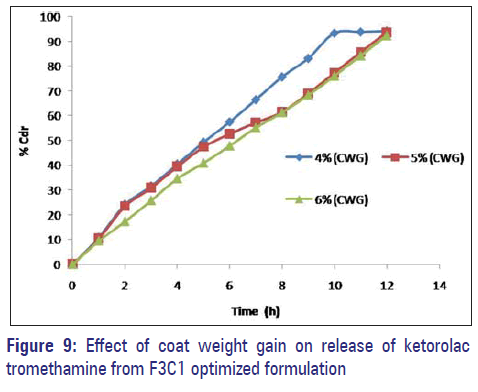
Effect of % weight gain
To study the effect of weight gain by coating on drug release, core tablets of ketorolac tromethamine were coated with coating composition C1 so as to get tablets with different weight gain (04%, 05%, and 06%). It is evident that drug release decreases with an increase in weight gain of the coating membrane and no burst effect was observed during the dissolution [Figure 9].
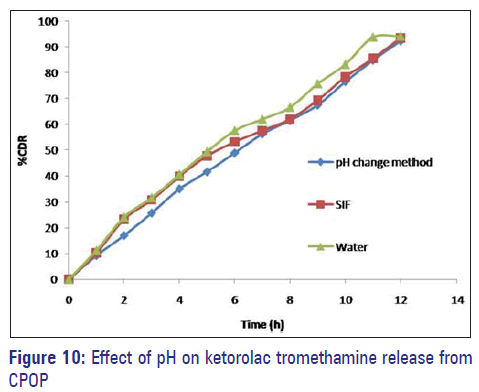
Effect of pH
In order to study the effect of pH and to assure a reliable in vivo performance, release studies of the optimized formulations F3C1 were conducted in media of different pH (SGF, pH 1.2, and SIF, pH 6.8), SIF, and water. Drug release profiles are superimposing [Figure 10]. Therefore the variations in pH of gastrointestinal tract had no effect on ketorolac tromethamine release.
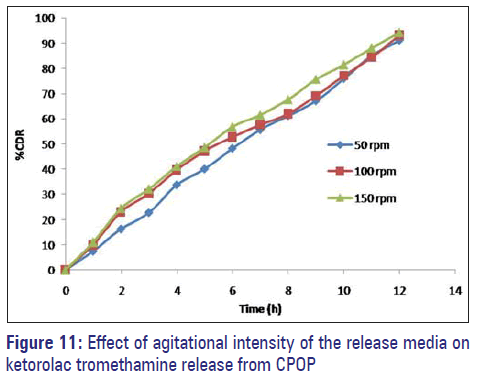
Effect of agitational intensity (RPM)
The release profile of ketorolac tromethamine from the F3C1 was fairly independent of the agitational intensity 50, 100, and 150 rpm of the release media, and hence, it can be concluded that the release was independent of the hydrodynamic conditions of the body [Figure 11].
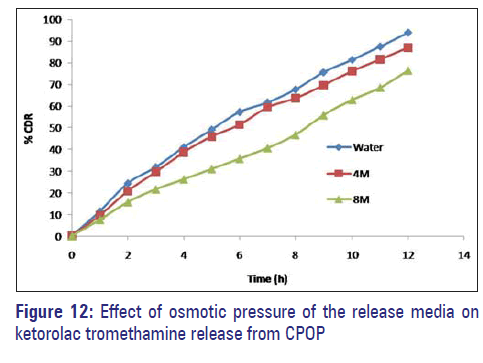
Effect of osmotic pressure
F3C1 formulation when exposed to media of different osmotic pressure showed that the drug release is highly dependent on the osmotic pressure of the release media. Ketorolac tromethamine release from the formulation decreased as the osmotic pressure of the media increased; hence the delivery system is dependent on osmotic pressure [Figure 12].
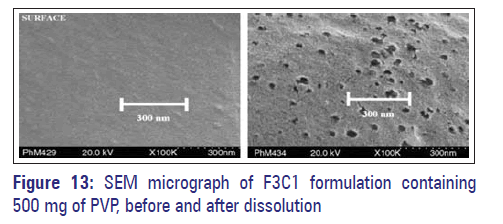
Scanning electron microscopy (SEM)
In order to elucidate the changes in the membrane structure, SEM studies were conducted (both before and after dissolution studies). Figure 13 shows SEM micrographs of coating membrane before and after dissolution of F3C1 optimized formulation containing 500 mg of PVP. The surface of the tablet was smooth before dissolution and after dissolution, the pores formed were in large number, and were visible, and a rough surface was observed on account of leaching [Figure 13]. Finally, it can be concluded that leaching of the pore former from the membrane had made membrane porous, through which drug release took place and designed drug delivery was controlled porosity osmotic pumps.
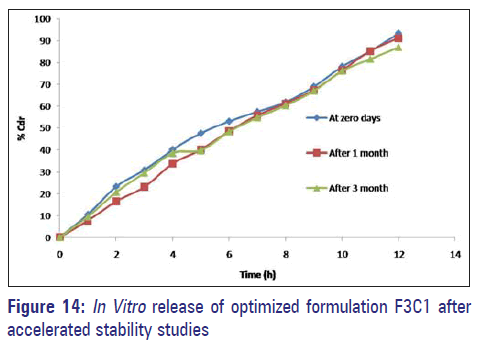
Accelerated stability study
After a period of 3 months, the formulations were found to be stable in terms of drug content and dissolution [Table 6 and Figure 14].
| At zero days | After 1 months | After 3 months |
|---|---|---|
| 97.1231% | 96.9335% | 96.8556 |
Table 6: Content uniformity of optimized controlled porosity osmotic pump F3C1 after accelerated stability studies.
Conclusion
A controlled porosity osmotic pump comprising a tablet coated with ethyl cellulose as a semipermeable membrane containing different levels of channeling agent has been developed for ketorolac tromethamine. The desired release profile was obtained by optimizing polymer concentration, channeling agent level, and plasticizer concentration. The release mechanism from F3C1 optimized formulation was based on osmotic pressure since release rate was significantly affected by the osmotic strength of the dissolution medium, directly proportional to the initial level of pore former, but inversely related to the membrane weight. Drug release from the developed formulations was found to be independent of pH and hydrodynamic conditions. Drug release from the developed formulations follows Peppas release kinetics and Fickian diffusion mechanism. SEM studies showed formation of pores in the membrane in the dissolution medium. Optimized formulation was found to be stable after 3 months of storage under accelerated stability conditions. CPOP was designed for effective administration of drugs for a prolonged period of time.
Acknowledgements
The authors are thankful to MSN Laboratories Ltd., Hyderabad. Symed Pharma Ltd., Hyderabad. Micro labs Pvt., Ltd., Bangalore, Elegant Drugs. Pvt. Ltd., Hubli and Standardcon Pvt. Ltd., Mumbai. for providing the gift samples to carry out research.
Source of Support: Nil, Conflfl ict of Interest: No.
References
- Shokri J, Ahmadi P, Rashidi P, Shahsavari M, Rajabi-Siahboomi A, Nokhodchi A. Swellable elementary osmotic pump (seop): An effective device for delivery of poorly water-soluble drugs. Eur J Pharm Biopharm 2008;68:289-97.
- Makhija SN, Vavia PR. Controlled porosity osmotic pump-based controlled release system of pseudoephedrine-I cellulose acetate as a semipermeable membrane. J Control Release 2003;89:5-18.
- Verma RK, Kaushal AM, Garg S. Development and evaluation of extended release formulations of isosorbide mononitrate based on osmotic technology. Int J Pharm 2003;263:9-24.
- Rao BP, Geetha M, Purushothama N, Sanki U. Optimization and development of swellable controlled porosity osmotic pump tablet for theophylline. Trop J Pharm Res 2009;8:247-55.
- Thombre AG, Appelb LE, Chidlaw MB, Daugherity PD, Evans LA, Sutton SC. Osmotic drug delivery using swellable-core technology. J Control Release 2004;94:75-89.
- Zenter GM, Rork GS, Himmelstein KJ. The controlled porosity osmotic pump. J Control Release 1985;1:269-82.
- Babu CA, Rao MP, Ratna V. Controlled-porosity osmotic pump tabletsan overview. J Pharm Res Health Care 2010;2:114-26.
- Carmelo P, Rosanna F, Antonella P, Paolo C, Luisa R, Francesco B, et al. Evaluation of alternative strategies to optimize ketorolac transdermal delivery. AAPS Pharm Sci Tech 2006;7:E1-8.
- Arora R, srivastava AK, Rani M, Mishra B. Investigation on osmotic matrix tablets as controlled delivery system of ketorolac tromethamine. Acta Pharm Turc 2002;44:87-96.
- Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm 2004;57:513-25.
- Rahman BM, Rahman MM, Ahmed M, Wahed MIB, Barman KR, Khan RA, et al. Development and evaluate the formulation of ketorolac tromethamine tablets and a comparative study with marketed product. Int J Med Sci 2007;2:102-5.
- Government of India, Ministry of health and family welfare, Indian Pharmacopoeia. Vol. 2. Ghaziabad: The Indian Pharmacopoeia Commission; 2010. p. 1543-46.
- Kanagale P, Lohray BP, Misra A, Davadra P, Kini R. Formulation and optimization of porous osmotic pump–based controlled release system of oxybutynin. AAPS Pharm Sci Tech 2007;8:E1-7.
- Liu H, Yang XG, Nie S, Wei L, Zhou L, Liu H. Chitosan-based controlled porosity osmotic pump for colon-specifi c delivery system: Screening of formulation variables and in vitro investigation. Int J Pharm 2007;332:115-24.
- Philip AK, Pathak K. In situ formed phase transited drug delivery system of ketoprofen for achieving osmotic, controlled and level a in vitro in vivo correlation. Indian J Pharm Sci 2008;70:745-53.