Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy
- *Corresponding Author:
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under th e identical terms.
Abstract
Objective: The rationale behind present research vocation was to develop and investigate a novel microsponge based gel as a topical carrier for the prolonged release and cutaneous drug deposition of fluconazole (FLZ); destined for facilitated fungal therapy. Materials and Methods: Microsponges were prepared using quasi‑emulsion solvent diffusion method using Eudragit S‑100. In the direction of optimization, the effect of formulation variables (drug‑polymer ratio and amount of emulsifier) and diverse factors affecting physical characteristics of microsponge were investigated as well. Fabricated microsponges were characterized by differential scanning calorimetry, Fourier transform‑infrared, scanning electron microscopy (SEM), particle size analysis, and also evaluated for drug content, encapsulation efficiency, in vitro drug release and in vitro antifungal activity. Results: Compatibility studies results reflected no sign of any chemical interaction between the drug and polymers used. Whereas, varied drug‑polymer ratios and emulsifier concentration indicated significant effect on production yield, drug content, encapsulation efficiency, particle size and drug release. Spherical microsponges with a porous surface and 29.327 ± 0.31 μm mean particle size were evident from SEM micrographs. In vitro release outcomes, from microsponge loaded gels depicted that F1 formulation was more efficient to give extended drug release of 85.38% at the end of 8 h, while conventional formulation by releasing 83.17% of drug got exhausted incredibly earlier at the end of 4 h merely. Moreover, microsponge gels demonstrated substantial spreadability and extrudability along with promising antifungal activity. Conclusions: Fabricated microsponges would be impending pharmaceutical topical carriers of FLZ and a leading alternative to conventional therapy for efficient, safe and facilitated eradication of fungal infections.
Keywords
Drug delivery, fluconazole, gel, microsponges, sustained release
Introduction
Topical treatment with fluconazole (FLZ) for severe life intimidating skin fungal infections has come out as an efficient therapy occupying a high-flying position among the alternatives of treatment. [1] Although, topical delivery of FLZ has resulted in systemic absorption and skin irritation and hence failed to accomplish mycological eradication. [2] Consequently, such problems lead to the poor patient compliance and compromising the efficacy of the therapy. Furthermore, in drug delivery, the topical administration of bioactive molecules is still a challenging area with the difficulty in controlling and determining the exact amount of drug reaching the different skin layers. [3,4] Furthermore, for the drug differential distribution in the various skin layers, the entrapped moiety, and the vehicle physicochemical characteristics contributes as well. [5,6] To conquer the renowned adverse effects, use of novel drug delivery systems has been proposed due to the potential of such systems to diminish such occurrences without affecting the efficacy. [7,8]
In recent times, for improving the bioavailability, sustained and controlled release of drug; quite a few new-fangled drug carrier systems have been developed for maintaining the localized effect as well as enhanced drug accumulation in various strata of skin, and the carrier systems includes liposomes and niosomes, [1] hydrogels, [2] lecithin-based organogel, [9] FLZ hydrogel, [10] polymeric mucoadhesive films, [11] and poly (lactide-co-glycolide) microspheres. [12] Up to now, a variety of difficulties arise with liposomes due to their stability issues. Besides, no large scale and satisfactory production method exists for microspheres. [7] Therefore, an interesting alternative approach for FLZ encapsulation may be the use of microsponge technology owing to its applicability for several drugs used in topical therapy of skin infections and diseases.
Further, poor water solubility of drugs is the major problem associated with transdermal delivery system causing difficulties during the formulation of conventional dosage forms. [13] One of the difficulties related to poor solubility is very less bioavailability and defective absorption. [14] Some formulation approaches adopted to overcome such difficulties include techniques, such as solubilization using co-solvents, micronization, augmented permeation using enhancers, surfactant dispersions, oily solutions, precipitation, and salt formation. [15] Other techniques such as emulsions, [16] microemulsions, [17] solid-dispersions, [18] and inclusion complexes using cyclodextrin [19] exemplify sensible success though they are short of global application for all drug moieties. For this reason, an easy approach carrying varied nature for resolving such problems is the need. [15,20,21] Moreover, most of the conventional topical products have been proposed to act on exterior layers of skin, which upon application releases their active components for fast absorption by producing a highly concentrated layer of actives. [22,23] Hence, a novel system is required which may increase the existence of active agents either on the skin surface or within the epidermis, alongside reducing transdermal penetration.
Microsponge delivery system (MDS) executes all these requirements and thereby giving an assurance of drug localization on the skin surface and within the epidermis without entering into systemic circulation to a large extent. The advantage of MDS includes the programmable release of drug and biological safety of the system. Furthermore, this technology offers quite a lot of benefits using drug entrapment such as better formulation flexibility, abridged side effects, improved elegance, and superior stability. [4,24-26] Antifungal drugs are well-established for their use in therapy of diverse bacterial and fungal infections. Numerous antifungals are available since the antiseptic era as topical dosage forms like gel, lotion, ointment and likely are also available as oral and parenteral products which could be used for treating topical (athlet’s foot, dermal candidiasis, dermatophytosis, fungal acne, face fungus etc.) and systemic (candidiasis, cryptococcosis, histoplasmosis, coccidioidomycosis, blastomycosis, aspergillosis, etc.) infections. To achieve therapeutic drug concentration of active agents in the target tissue by simultaneously keeping the systemic and gastrointestinal concentrations as low as possible to avoid unwanted effects; it becomes preferable to deliver the drug into the body via a route other than the oral. Taking into consideration adverse drug reactions allied with oral formulations and expectation of quickly localized effect, many antifungal drugs are administered by topical route. [8,27-29] The proficient and safe topical formulation containing antifungal agent has remarkable and wide applications for treating a range of disseminated fungal infections owing to the facts; elimination of intravenous route allied acute toxicity (chilling and fever), diminished renal toxicity, no or shorten hospitalization, reduced dose frequency, and increase patient compliance. [30,31]
So, by contemplating these beneficial facts of MDS, drawbacks of conventional topical dosage forms, and lack of availability of microsponge based delivery system of FLZ in the market; present research work was aimed to formulate and evaluate a novel microsponge based gel of FLZ for its promising effects in eradication of fungal infections over extended period of time.
Materials and Methods
The materials required for the present work were procured from diverse sources. FLZ was procured from FDC Ltd., Mumbai, India and Eudragit S-100 was provided as gift sample by Evonik Pharma, Mumbai, India. Polyvinyl alcohol (PVA) and dibutyl phthalate were procured from Central Drug House Pvt. Ltd., Mumbai, India and Qualigens Fine Chemicals, Mumbai, India, respectively. All the other ingredients used were of analytical grade and were used as procured.
Characterization of pure drug
Melting point
The melting point of FLZ was noted by micro controlled based melting point apparatus (SMP10/1, Stuart, UK).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) study was carried out to evaluate thermal behavior and thermo tropic characteristics of the drug. Nearly 5 mg sample was sealed in aluminum pan followed by heating at a rate of 10°C/min over temperature range of 40–200°C under nitrogen atmosphere of flow rate 10 ml/min and thermogram (Mettler-Toledo DSC 821e, Switzerland) was obtained.
Fourier transform-infrared spectroscopy
Infrared (IR) spectra were recorded to check purity of drug and excipients. It was determined by fourier transform-IR (FT-IR) spectrophotometer (FT-IR, Shimadzu 8400 S, Japan) over the wavelength range of 4000–400 cm-1 at a resolution of 4 cm-1. [32-34] Samples were dispersed in KBr and compressed in pellets by applying five tons pressure for 5 min using a hydraulic press. Formed pellets were kept in the light path and spectra were recorded.
Ultraviolet-visible spectroscopy
Calibration curve of FLZ was plotted using phosphate buffer solution (PBS) of pH 7.4 by keeping concentration range of 5–40 µg/ml. The drug was analyzed spectrophotometrically (Pharmaspec 1700, Shimadzu, Japan) at 260 nm (regression coefficient r2 = 0.993).
Drug-excipient interaction study
The drug-excipient interaction was investigated by FT-IR and DSC studies. IR spectra were recorded to check compatibility of the drug with excipients. DSC gives an idea regarding physical properties of the sample nature (crystalline or amorphous) and indicates any probable interaction among drug and excipients.
Preparation of fluconazole microsponges
The microsponges containing FLZ were fabricated by quasi-emulsion solvent diffusion method [27,32,35] using an inner phase comprising Eudragit S-100 and dibutyl phthalate (1% w/v) dissolved in 5 ml of ethanol:dichloromethane (1:1). Dibutyl phthalate was added to improve the plasticity of the polymer. Further FLZ was put in and dissolved through ultrasonication at 35°C. This mixture was then poured into an aqueous solution of PVA (outer phase) with stirring rate 500 rpm for 60 min. Next on, microsponges were formed due to the removal of dichloromethane and ethanol from the system by evaporation. Prepared microsponges were then filtered, washed with distilled water and subjected to drying at 40°C for 12 h in hot air oven. Finally, microsponges were weighed to determine production yield. Various formulation batches are prepared as per Table 1.
| Ingredients | Formulation batches | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| Fluconazole: Eudragit S-100 (mg) | 1:1 | 1:2 | 1:3 | 1:4 | 1:5 | 1:6 | 1:3 | 1:3 | 1:3 | 1:3 |
| Ethanol: dichloromethane (ml) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dibutyl phthalate (% w/v) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Polyvinyl alcohol (mg) | 50 | 50 | 50 | 50 | 50 | 50 | 30 | 40 | 60 | 70 |
| Water (ml) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 1: Composition of fluconazole microsponges
Evaluation of fluconazole microsponges
Production yield
Production yield of microsponges was determined by formula mentioned below. [36]
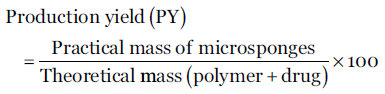 Eq. (1)
Eq. (1)
Actual drug content and encapsulation efficiency
Precisely weighed quantity (100 mg) of microsponges containing drug was kept in 100 ml PBS (pH 7.4) for 12 h with continuous stirring. Samples were filtered using 0.45 µm membrane filter and further analyzed at 260 nm next to blank using ultraviolet-visible (UV) spectrophotometer (Pharmaspec 1700, Shimadzu, Japan). Estimation of drug content and encapsulation efficiency for all batches were done using following expressions. [33]
 Eq. (2)
Eq. (2)
 Eq. (3)
Eq. (3)
Where Mact = actual FLZ content in weighed quantity of microsponges, Mms = weighed quantity of microsponges and Mthe = theoretical FLZ content in microsponges.
Scanning electron microscopy
For morphology and surface topography, prepared microsponges were examined with a scanning electron microscope (LEO 440i, UK) operating at 5 kV. Using double adhesive tape, samples were mounted on a metal stub and coating with platinum/palladium alloy under vacuum was done. [37]
Particle size analysis
Particle size analysis of prepared microsponges was studied by using particle size analyzer (Malvern Mastersizer Hydro 2000, Malvern, UK). Microsponges were dispersed in double distilled water before running the sample in the instrument to ensure that light scattering signal (as indicated by particles count per second) was within the sensitivity range of the instrument. The analysis was carried out at room temperature, keeping the angle of detection at 90°. The average particle size was expressed in terms of d (0.9) μm. [6]
Thermal analysis
Thermogram of FLZ microsponge formulation was obtained using differential scanning calorimeter (Mettler-Toledo DSC 821e, Switzerland) outfitted with an intercooler. For calibrating DSC enthalpy and temperature scale, indium standard was implied. Microsponge samples were kept in aluminum pan hermetically and heated at a constant rate of 10°C/min over a temperature range of 40–200°C. By purging nitrogen with a flow rate of 10 ml/min inert atmosphere was maintained.
Infrared spectroscopy
Using FT-IR spectrophotometer (FT-IR, Shimadzu 8400 S, Japan) implying KBr pellet method, spectra of FLZ, physical mixtures of FLZ and Eudragit S-100, and microsponge formulation were recorded in the wavelength range of 4000–400 cm-1.
X-ray diffraction study
X-ray diffraction patterns were recorded by using X-ray diffractometer (Seimens, Model D5000, Germany) with CuKα radiation of wavelength 1.5405 A° and a crystal monochromater. The instrument was operated at voltage 45 mV and current 20 amp. Diffraction patterns were run at 5–10°C/min in terms of 2 θ; crystal and physical state of FLZ were characterized.
Preparation of fluconazole microsponge gel
Forming polymer (Carbopol 940) was soaked in water for 2 h and then dispersed by agitation at approximately 600 rpm with the aid of magnetic stirrer to get a smooth dispersion. The dispersion was allowed to stand for 15 min to expel entrained air. To it the aqueous solution of triethanolamine (2% v/v) was added with slow agitation for adjusting pH to 6.5–7.5. [24,38] At this stage permeation enhancers and microsponges containing drug equivalent to marketed formulation were incorporated into the gel base. Prepared gels were packed in wide mouth glass jar covered with screw capped plastic lid after covering the mouth with aluminum foil and were kept in dark and cool place until use.
Evaluation of fluconazole microsponge gel
Visual inspection
The organoleptic properties, such as color, texture, consistency, homogeneity, and physical appearance of gel containing microsponges were checked by visual observation.
pH measurement
Gel formulation pH was recorded using digital pH meter. 5 g gel was dispersed in 45 ml distilled water at 27°C and solution pH was measured. [24]
Spreadability studies
One of the requisite qualities for an ideal gel is to pursue excellent spreadability. Spreadability is used to express the extent of the area of skin or affected part to which gel readily spreads. A spreading value significantly affects therapeutic efficacy of the formulation. Expression of spreadability is given in terms of time (in seconds) taken by two slides to slip off from gel placed in between under application of specific load. Better spreadability is indicated by minimum time required for slides separation. Mathematical expression used for spreadability calculation was:
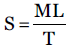 Eq. (4)
Eq. (4)
Where, M = weight (in gm) attached to upper slide, L = length (in cm) of glass slides, T = time (in sec) taken to separate the slides.
Wooden block-glass slides apparatus was used and by applying weight about 20 gm, time for complete separation of the upper slide (movable) from lower slide (fixed) was estimated.
Tube extrudability
One of the properties which an ideal gel should possess is good tube extrudability; so that when slight pressure is applied on tube, the formulation should extrude out uniformly with an ease. The technique adopted for examining gel extrudability was based upon percent quantity of gel extruded from the tube on finger pressure application. More the quantity extruded better the extrudability. Formulations were filled in clean, lacquered, collapsible aluminum tubes with 5 mm nasal tip opening and the pressure was applied on tubes using the first finger and thumb. Afterward tube extrudability was estimated in percentage by measuring the amount of gel extruded through the tip and compared with marketed formulation considering its extrudability as 100%. [39]
Viscosity
The viscosity of the gel formulation was measured with a Brookfield viscometer (Brookfield, USA; Capcalc Version 2.2) using 1x model and cone number 01, with an angular velocity of 5 rpm at 25 ºC. An average of five readings was used to calculate viscosity.
In vitro release studies
The in vitro release of gel formulations were studied using Franz diffusion cells. The cellophane membrane (pore size 0.45 µm) was mounted onto diffusion cell with 37 ml receptor compartment volume. PBS (pH 7.4) was used as receptor medium, and the system was thermostated to 37 ± 1°C under stirring. All batches of drug microsponge gel (F1-F10) and marketed formulation (F11) were subjected to the diffusion study. Aliquots of 1 ml volume were withdrawn at specific time intervals by maintaining sink condition simultaneously. Withdrawn aliquots were then diluted using receptor medium and analyzed by UV spectrophotometer (Pharmaspec 1700, Shimadzu, Japan) at 260 nm against PBS pH 7.4. To resolve drug release mechanism and to compare release profiles disparities amongst formulations, data obtained from drug release and time was used. Further, release data was analyzed using diverse mathematical models to know release kinetics. [38]
In vitro antifungal activity
In vitro antifungal activity of FLZ microsponge optimized batch (F1) gel was carried out by Cup-plate method in comparison with FLZ marketed gel (F11, Flucos 0.5% gel) as standard. Fungi cultures used for biological evaluation were Candida albicans MTCC 1637. [27,40]
Stability study
Optimized gel formulation was subjected to stability testing as per ICH norms. Gel was filled in clean, lacquered, collapsible aluminum tubes, and various replicates were kept at 40 ± 2°C and 75 ± 5% relative humidity in a humidity chamber. Gel was assessed for change in appearance, pH and in vitro release profile at an interval of 30, 60 and 90 days.
Results and Discussion
Characterization of pure drug
Melting point
The melting point of FLZ was experiential in the range of 139-141°C (Literature standard 138–142°C). As experimental values were in good agreement with standard, procured drug was supposed to be pure.
Differential scanning calorimetry
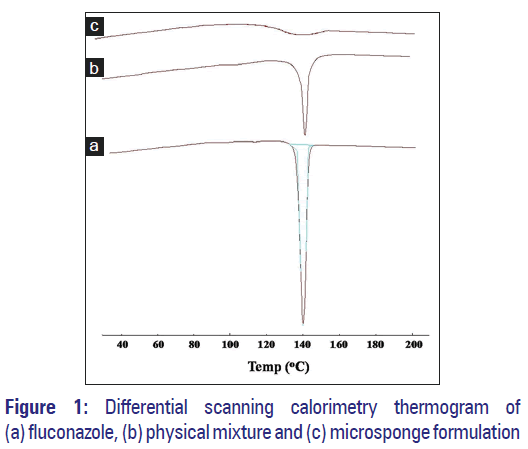
As portrayed by thermogram shown in Figure 1a, a sharp endothermic peak was observed at 140°C with respect to the melting point of the drug; reflecting drug purity.
Fourier transform-infrared spectroscopy
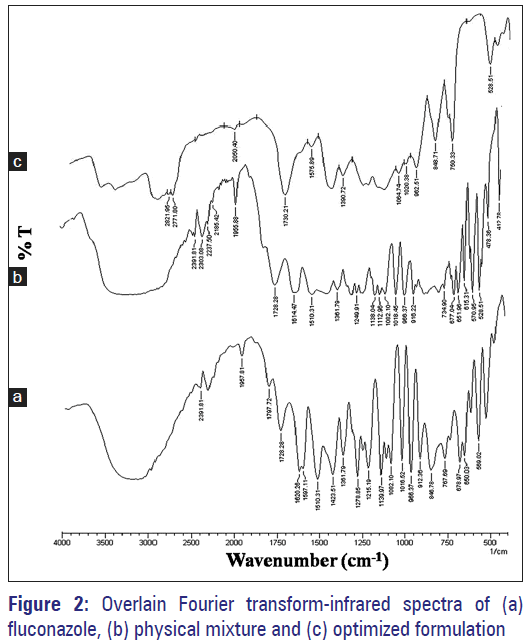
The infrared spectrum of drug sample was recorded [Figure 2a] and spectral analysis was done. The characteristics IR absorption peaks of FLZ at 1193–1062 cm-1 (C-O bending), 1278–1215 cm-1 (C-F stretch), 1620–1507 cm-1 (C = C stretch), were present in procured drug sample spectrum; which confirmed the purity.
Drug-excipient interaction study
Compatibility study was carried out to check for any possible interaction between drug and excipients used. It was performed using FT-IR and DSC. In DSC studies, pure FLZ thermogram reflected an endothermic peak at 140.22°C corresponding to its melting point [Figure 1a]. Physical mixture showed similar thermal behavior as that of the pure drug but with lower intensity [Figure 1b]. However, the melting endotherm of microsponge formulation was suppressed corresponding to the partial protection of FLZ since microsponge encapsulation [Figure 1c]. It was also observed that drug crystallinity altered significantly in microsponge formulation confirming its dispersion in the system.
FT-IR spectroscopic study results discovered no any new peak appearance or disappearance of existing peaks, discarding any chemical interaction probability among drug and polymer used [Figure 2a]. All characteristic peaks of FLZ were experiential in the physical mixture and microsponge formulation spectrum [Figure 2b and c]. Thus, IR spectroscopy results depicted that drug was compatible with selected polymer, excipients and possess good stability in all microsponge formulations.
Evaluation of fluconazole microsponges
Physical appearance
White to almost white, microsponge particles were obtained by quasi-emulsion solvent diffusion method.
Production yield
The production yield of all batches of microsponges ranged from 23.35% to 79.75% [Table 2]. Drug: Polymer ratio and PVA concentration were found to affect the production yield significantly. In the case of drug: Polymer ratio 1:1 (F1), production yield was very low, i.e. 23.35% while for drug: Polymer ratio 1:6 (F6), it was 79.75%.
| Batch code | Drug: polymer ratio | Actual drug content (%)±SD* | Encapsulation efficiency (%)±SD* | Production yield (%)±SD* | Percentage CDR±SD* | Flux (mg/cm2/h) | ||
|---|---|---|---|---|---|---|---|---|
| F1 | 1:1 | 45.28±0.01 | 96.56±0.02 | 23.35±0.02 | 85.38±0.01 | 0.3225 | ||
| F2 | 1:2 | 31.47±0.02 | 94.41±0.01 | 42.76±0.05 | 77.38±0.03 | 0.2923 | ||
| F3 | 1:3 | 22.50±0.03 | 88.82±0.02 | 51.25±0.02 | 65.76±0.02 | 0.2483 | ||
| F4 | 1:4 | 16.86±0.01 | 84.32±0.20 | 69.10±0.11 | 57.36±0.05 | 0.2166 | ||
| F5 | 1:5 | 13.32±0.01 | 80.09±0.00 | 75.33±0.28 | 48.89±0.01 | 0.1846 | ||
| F6 | 1:6 | 10.25±0.02 | 74.57±0.03 | 79.75±0.02 | 42.37±0.01 | 0.1600 | ||
| F7 | 1:3 | 30.25±0.15 | 90.75±0.04 | 34.33±0.49 | 80.67±0.00 | 0.3047 | ||
| F8 | 1:3 | 31.78±0.02 | 95.50±0.02 | 39.66±0.47 | 78.25±0.01 | 0.2956 | ||
| F9 | 1:3 | 32.01±0.01 | 96.30±0.04 | 48.10±0.61 | 76.32±0.02 | 0.2883 | ||
| F10 | 1:3 | 32.15±0.21 | 96.15±0.04 | 50.66±0.51 | 74.35±0.00 | 0.2809 | ||
| *Standard deviation mean “n”=3. CDR: Cumulative drug release, SD: Standard deviation | ||||||||
Table 2: Actual drug content, encapsulation efficiency, production yield and percentage cumulative drug release
It reflected that higher the drug: Polymer ratio, higher the productions yield. Further, with low PVA concentration (30 mg, F7), production yield was quite low, i.e., 34.33% and as the concentration was increased from 30 mg to 70 mg, the production yield was also found to be increase. The reason for the increase in production yield at an elevated drug: Polymer ratio was abridged dichloromethane diffusion rate from concentrated solutions to the aqueous phase, which provides additional time for formation of droplet following improved yield.
Actual drug content and encapsulation efficiency
The mean amount of drug entrapped in fabricated microsponges was found to be lesser than theoretical value for every drug: Polymer ratio, because drug encapsulation efficiency did not attain 100%. It was because of some drug dissolution in aqueous phase or solvent used. Encapsulation efficiency outcomes reflected that higher drug: Polymer ratios led to superior drug loadings. Incorporation of higher PVA amounts during the preparation of microsponges at an elevated drug: Polymer ratio caused slight increase in dispersed phase viscosity. On the diffusion of solvents from inner phase, almost all of dispersed phase was transformed to solid microsponges and estranged particles emerged. The utmost drug loading efficiencies of these formulations could be attributed to the availability of maximum polymer amount to each drug unit in contrast to rest of the formulations. The encapsulation efficiencies were in the range of 74.57–96.56% as quoted in Table 2.
Scanning electron microscopy
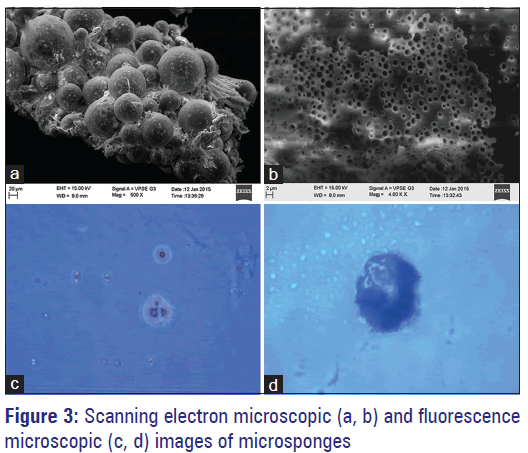
Prepared microsponges were subjected to scanning electron microscopy (SEM) analysis for assessing their morphology and surface topography. The captured SEM images of microsponges are shown in Figure 3a and b. SEM photomicrographs reflected that microsponges formed were highly porous, predominantly spherical and not much entire FLZ crystals were observed visually. By diffusion of solvent from the surface of microsponges, pores were induced. Moreover, it was exposed that the distinctive internal structure comprised of spherical cavity enclosing a stiff shell assembled of drug and polymer. The microsponges were also observed under fluorescence microscope [Figure 3c and d], which revealed that formed microsponges were spherical as each single entity and had porous nature.
X-ray diffraction study
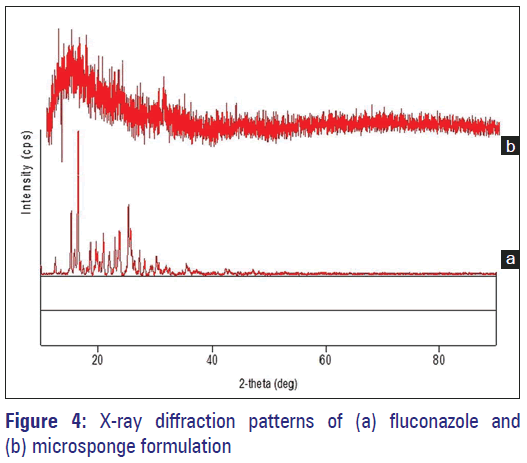
To obtain information on the physicochemical characteristics of the prepared microparticles, X-ray powder diffraction measurements were conducted. In the X-ray diffractogram sharp peaks at a diffraction angle (2 θ) 15.3, 16.6, 18.7, 19.7, 21.0, 23.8, 25.4, 25.7, and 27.4 were obtained in the case of FLZ and its microsponge formulation [Figure 4a and b]. Moreover, X-ray powder diffraction has been a useful method for determining the presence of polymorphs or crystal habit modifications in drug crystals. In general, for two forms of crystals, when the patterns (i.e., peak positions) are identical, they have the same internal structures; whereas if the patterns are different, then the crystals have different internal structures and are polymorphs. Here, all the samples exhibited spectra with similar peak positions (2 θ values). Therefore, the probability of the presence of different polymorphs of FLZ in these samples was ruled out.
Particle size analysis
The average particle size of microsponges should be in the range of 5–300 µm. The visual inspection of all batches using the optical microscope for particle size revealed that particle size has increased with increase in drug: Polymer ratio. It was because of the fact that polymer available at a higher drug: Polymer ratio was in more amount thereby increasing polymer wall thickness which led to the greater size of microsponges. Also with increasing amount of PVA, particle size was found to be increase; this was due to rise in apparent viscosity at augmented PVA concentrations. It results in bigger emulsion droplets formation, and consequently in larger microsponge size. [6,27] Optimized batch (F1) possessed more percent of intact, uniform, spherical particles during optical microscopy; hence subjected to analysis using photon correlation spectroscopy (Malvern Mastersizer Hydro 2000, Malvern, UK). The results revealed particle size d (0.9) corresponding to 29.327 ± 0.31 µm.
Evaluation of fluconazole microsponge gel
Visual inspection
The prepared formulations were inspected visually for their color, texture and appearance. All developed preparations were much clear and transparent with good homogeneity and absence of any lumps or syneresis.
pH
The pH values of all prepared formulations were found to be in the range of 6.3–6.9, which are supposed to be suitable to pass up threat of nuisance on application to skin.
Extrudability study
The extrudability of prepared microsponge gel was found to be 96.72% by considering extrudability of pure unentrapped drug gel as 100%. So, the prepared formulation exhibited better extrudability which is one of the ideal characteristics a gel should possess.
Spreadability study
The findings of spreadability depicted that formulated gel get easily spread on applying small amount of shear. Spreadability of gel containing pure drug and drug microsponges was found to be 3.84 gm.cm/sec and 4.25 gm.cm/sec respectively; indicating that spreadability of drug loaded microsponge gel was superior as compared to that of marketed one.
Viscosity
The viscosity of optimized FLZ microsponge gel formulation was found to be 2.25 Pa.s.
In vitro drug release
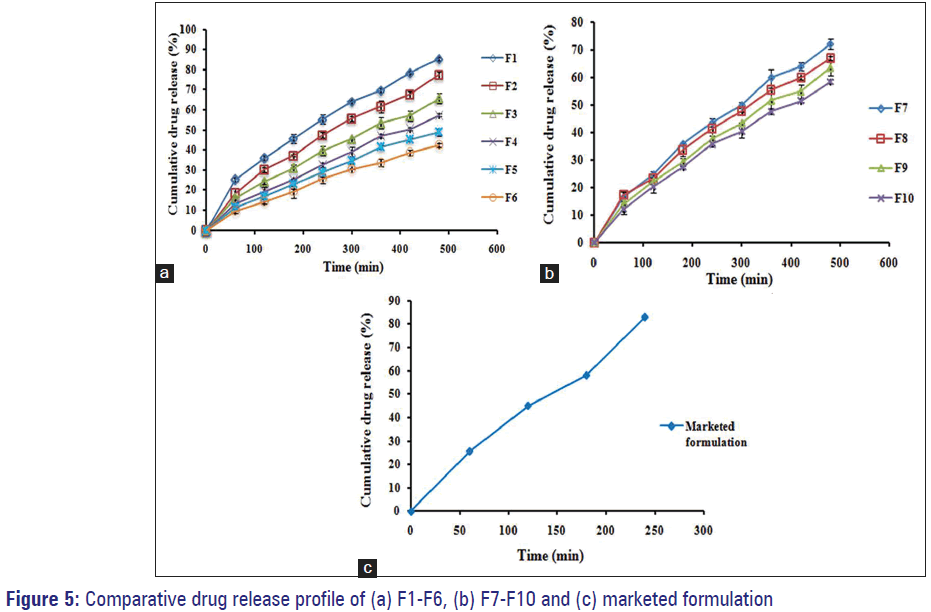
The drug release was observed to decline within a range of 85.38–42.37% with respect to rise in drug: Polymer ratio from 1:1 to 1:6. This is because as drug: Polymer ratio has increased; for each microsponge, to encapsulate drug, the polymer amount available was more. So, the thickening of polymer matrix wall results in extended diffusion path and ultimately led to lesser drug release. The highest drug release, i.e. 85.38% was found for the formulation F1, while the lowest 42.37% for F6. Initial burst release observed for formulations F1 and F2 (i.e. 25.48% and 18.50%, respectively); can be owed to the existence of nonencapsulated drug near or on the exterior of microsponges. Graphical presentation for comparative drug release of all batches (F1-F6) and (F7-F10) are shown in Figure 5a and 5b respectively.
It has been noted that for each formulation from F7 to F10, the drug release went to decrease with increasing amount of PVA. This could be attributed to the fact that the polymer matrix releases drug after complete swelling of polymer and the time required for swelling of the polymer is directly proportional to polymer amount in the formulation. The slight decrease in release rate with increased amount of PVA was from 72.35% to 58.67% for formulations F7-F10.
Release profile of marketed fluconazole gel
The drug release study for gel containing pure, unentrapped FLZ (marketed formulation, F11) was carried out and release profile obtained was as depicted in Figure 5c. The gel released 83.17% drug at the end of 4 h only and got exhausted. In contrast, gel containing entrapped drug in microsponges released the drug slowly up to 8 h thereby minimizing the side effects, skin irritation, and hypersensitivity reactions. As the F1 formulation exhibited drug release 85.38% after completion of 8 h, and also found superior in terms of physiochemical characterization, production yield, actual drug content, entrapment efficiency, morphology, surface topography, particle size, percent intact porous microsponges, and other physical parameters, in addition to drug release; it has implicit that F1 formulation was best and more efficient to give an extended drug release among the all formulations. [6,24]
Release kinetics
The in vitro release data were subjected to various release models namely, zero order, first order, Higuchi, Peppas and Hixson–Crowell, and best fit model was decided by highest r2 value. The in vitro drug release showed highest regression value for the zero order model (0.999 for F6). On the basis of maximum regression value, zero order was found to be the best fit model for most of the formulations [Table 3].
| Batch code | Zero order | First order | Higuchi | Peppas | Hixson–Crowell | Best fitting model |
|---|---|---|---|---|---|---|
| F1 | 0.996 | 0.980 | 0.991 | 0.956 | 0.956 | Zero order |
| F2 | 0.978 | 0.983 | 0.967 | 0.967 | 0.981 | First order |
| F3 | 0.976 | 0.944 | 0.935 | 0.954 | 0.922 | Zero order |
| F4 | 0.964 | 0.911 | 0.905 | 0.962 | 0.965 | Hixson–Crowell |
| F5 | 0.914 | 0.995 | 0.970 | 0.994 | 0.981 | First order |
| F6 | 0.999 | 0.994 | 0.964 | 0.995 | 0.989 | Zero order |
| F7 | 0.997 | 0.992 | 0.980 | 0.967 | 0.967 | Zero order |
| F8 | 0.971 | 0.993 | 0.969 | 0.986 | 0.975 | First order |
| F9 | 0.983 | 0.969 | 0.956 | 0.980 | 0.981 | Zero order |
| F10 | 0.979 | 0.972 | 0.945 | 0.958 | 0.976 | Zero order |
Table 3: Release kinetics data of microsponge formulations
Effect of formulation variables
Effect of drug: Polymer ratio
It has been observed that an increase in drug: Polymer ratio resulted as an increase in production yield; while drug content, encapsulation efficiency and percent drug release were found to be decreased [Table 2]. This is due to the fact that as a drug: Polymer ratio went on increasing, the polymer amount available for each micro sponge to encapsulate the drug was more, thus rising polymer matrix wall thickness which led to extended diffusion path and ultimately to lesser drug release. As a result, the amount of drug diffused and flux of the formulations was decreased at a higher drug: Polymer ratio.
Effect of composition of external phase
By changing the concentration of PVA from 30 to 70 mg effect of external phase composition was assessed for formulations F7-F10. It was observed that on increasing the amount of PVA, production yield, encapsulation efficiency and particle
In vitro antifungal activity
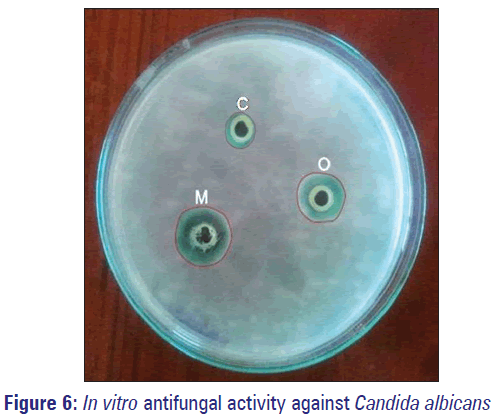
In vitro antifungal activity results for optimized FLZ microsponge gel (O) and marketed formulation (M), against fungal strains of C. albicans (causative organism for fungal acne, face fungus, candidiasis etc.) are as indicated in Table 4 and Figure 6. From experimental outcomes, it was concluded that prepared microsponges based gel formulation exhibited promising antifungal activity.
| Sample code | Zone of inhibition diameter (mm)±SD* |
|---|---|
| C (−ve control) | 11±0.21 |
| O | 21±0.14 |
| M | 24±0.11 |
*Standard deviation mean “n”=3. SD: Standard deviation
Table 4: Observations of in vitro antifungal activity
Stability study
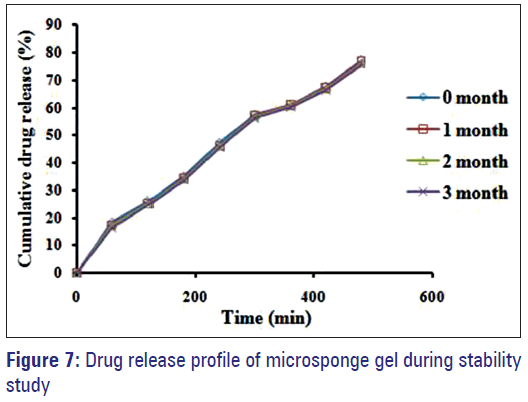
During stability study formulation appearance was found pearl white, homogenous, smooth and no significant deviation in pH was seen. Moreover, there were no considerable changes in drug content as well as percent drug release. Therefore, no evidence of drug degradation was observed.
After comparison of drug release profiles of optimized formulation F1 before and after 3 months stability study, similarity factor (f2) was calculated [Figure 7]; which was found to be f2 = 84.79 (>50). As similarity factor >50 indicates good stability of the product, in view of this it was concluded that the formulation was stable over the period of 3 months.
Conclusions
Polymeric microsponge based system of FLZ was developed successfully using quasi-emulsion solvent diffusion method for continual topical delivery up to an extended period so as to reduce application frequency, hypersensitive reactions allied to the conventional marketed formulation, and to improve bioavailability and safety. Implemented method was found to be simple, reproducible and rapid; which led to the formation of highly porous, spherical microsponges with good flow. Varied drug-polymer ratio reflected remarkable effect on drug content, encapsulation efficiency, particle size, and drug release. Among the all prepared gels integrating FLZ-loaded microsponges, the F1 formulation was chosen for further study on the basis of its superiority in terms of physiochemical characterization, production yield, drug content, entrapment efficiency, morphology, surface topography, intact particles percent, and particle size. The in vitro drug release study outcomes showed highest regression values for the zero order model, and also established proficiency of F1 formulation for extended drug release (85.38% at 8 h) with respect to conventional marketed one. The in vitro antifungal evaluation and stability study too depicted promising results. Thus, microsponge based delivery system developed and investigated in present research approach was seems to be promising with respect to eradication of face fungus, candidiasis and numerous other fungal infections, along with the practical application in pharmaceuticals and cosmeceuticals.
Acknowledgements
Authors are grateful to the Dean, College of Pharmacy, University of Hail, Hail, Saudi Arabia for his constructive suggestion and timely help throughout the work. The authors express their heartfelt gratitude toward FDC Ltd., Mumbai, India and Evonik Pharma, Mumbai, India for providing the gift samples of FLZ and Eudragit S-100 respectively. Furthermore, we thank the Managing Authorities of JSS College of Pharmacy and JSS University, Mysuru for furnishing obligatory facilities to complete the research work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, et al. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. J Liposome Res 2010;20:341-50.
- Bidkar S, Jain D, Padsalg A, Patel K, Mokale V. Formulation development and evaluation of fluconazole gel in various polymer bases. Asian J Pharm 2007;1:63-8.
- Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev 2007;59:427-43.
- Osmani RA, Aloorkar NH, Kulkarni AS, Harkare BR, Bhosale RR. A new cornucopia in topical drug delivery: Microsponge technology. Asian J Pharm Sci Tech 2014;4:48-60.
- Teichmann A, Heuschkel S, Jacobi U, Presse G, Neubert RH, Sterry W, et al. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm 2007;67:699-706.
- Pawar AP, Gholap AP, Kuchekar AB, Bothiraja C, Mali AJ. Formulation and evaluation of optimized oxybenzone microsponge gel for topical delivery. J Drug Deliv 2015;2015:261068.
- Castro GA, Coelho AL, Oliveira CA, Mahecha GA, Oréfice RL, Ferreira LA. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int J Pharm 2009;381:77-83.
- Bothiraja C, Gholap AD, Shaikh KS, Pawar AP. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv 2014;5:781-94.
- Jadhav KR, Kadam VJ, Pisal SS. Formulation and evaluation of lecithin organogel for topical delivery of fluconazole. Curr Drug Deliv 2009;6:174-83.
- Abdel-Mottaleb MM, Mortada ND, El-Shamy AA, Awad GA. Physically cross-linked polyvinyl alcohol for the topical delivery of fluconazole. Drug Dev Ind Pharm 2009;35:311-20.
- Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole mucoadhesive buccal films: In vitro/in vivo performance. Curr Drug Deliv 2009;6:17-27.
- Rivera PA, Martinez-Oharriz MC, Rubio M, Irache JM, Espuelas S. Fluconazole encapsulation in PLGA microspheres by spray-drying. J Microencapsul 2004;21:203-11.
- Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The microsponge delivery system of benzoyl peroxide: Preparation, characterization and release studies. Int J Pharm 2006;308:124-32.
- Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci 2003;18:113-20.
- Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm 2006;317:61-8.
- Riaz M. Review article: Stability and uses of liposomes. Pak J Pharm Sci 1995;8:69-79.
- Jadhav KR, Shaikh IM, Ambade KW, Kadam VJ. Applications of microemulsion based drug delivery system. Curr Drug Deliv 2006;3:267-73.
- Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 2000;50:47-60.
- Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005;6:E329-57.
- Jain V, Singh R. Dicyclomine-loaded Eudragit®-based microsponge with potential for colonic delivery: Preparation and characterization. Top J Pharm Res 2010;9:67-72.
- Srivastava R, Kumar D, Pathak K. Colonic luminal surface retention of meloxicam microsponges delivered by erosion based colon-targeted matrix tablet. Int J Pharm 2012;427:153-62.
- Kaity S, Maiti S, Ghosh AK, Pal D, Ghosh A, Banerjee S. Microsponges: A novel strategy for drug delivery system. J Adv Pharm Technol Res 2010;1:283-90.
- Sharma R, Roderick B, Walker R, Pathak K. Evaluation of the kinetics and mechanism of drug release from econazole nitrate nanosponge loaded carbapol hydrogel. Indian J Pharm Educ Res 2011;45:25-31.
- Osmani RA, Aloorkar NH, Ingale DJ, Kulkarni PK, Hani U, Bhosale RR, et al. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J 2015;23:562-72.
- D’souza JI, More HN. Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. Res J Pharm Technol 2008;1:502-6.
- Vyas LK, Tapar KK, Laddha BH, Lahoti AO, Nema RK. Formulation and development of anti-blemish preparations using microsponge technology. J Chem Pharm Res 2010;2:562-71.
- Osmani RA, Aloorkar NH, Kulkarni AS, Kulkarni PK, Hani U, Thirumaleshwar S, et al. Novel cream containing microsponges of anti-acne agent: Formulation development and evaluation. Curr Drug Deliv 2015;12:504-16.
- Partibhan KG, Manivannan R, Krishnarajan D, Chandra S, Nidhin R. Microsponges role in novel drug delivery. Int J Pharm Res Dev 2011;3:117-25.
- Srilakshmi P, Srinivas MP. Development and evaluation of voriconazole microsponges for topical delivery. Inventi Rapid: Pharm Tech 2011;2011:75-81.
- Bhanu VP, Shanmugam V, Lakshmi PK. Development and optimization of novel diclofenac emulgel for topical drug delivery. Int J Compd Pharm 2011;9:1-4.
- Shaikh KS, Pawar AP. Liposomal delivery enhances cutaneous availability of ciclopiroxolamine. Lat Am J Pharm 2010;29:763-70.
- Osmani RA, Aloorkar NH, Thaware BU, Kulkarni PK, Moin A, Hani U, et al. Microsponges based drug delivery system for augmented gastroparesis therapy: Formulation development and evaluation. Asian J Pharm Sci 2015;10:442-51.
- Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 2006;318:103-17.
- Li SS, Li GF, Liu L, Jiang X, Zhang B, Liu ZG, et al. Evaluation of paeonol skin-target delivery from its microsponge formulation: In vitro skin permeation and in vivo microdialysis. PLoS One 2013;8:e79881.
- Comoglu T, Gönül N, Baykara T. The effects of pressure and direct compression on tabletting of microsponges. Int J Pharm 2002;242:191-5.
- Kiliçarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm 2003;252:99-109.
- Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR. Factors affecting the morphology of benzoyl peroxide microsponges. Micron 2007;38:834-40.
- Zaki Rizkalla CM, latif Aziz R, Soliman II. In vitro and in vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS PharmSciTech 2011;12:989-1001.
- Purushothamrao K, Khaliq K, Sagare P, Patil SK, Kharat SS, Alpana K. Formulation and evaluation of vanishing cream for scalp psoriasis. Int J Pharm Sci Technol 2010;4:32-41.
- Saboji JK, Manvi FV, Gadad AP, Patel BD. Formulation and evaluation of ketoconazole microsponge gel by quasi-emulsion solvent diffusion. J Cell Tissue Res 2011;11:2691-6.