Evaluation of anxiolytic effects of aripiprazole and hydroxyzine as a combination in mice
- *Corresponding Author:
- Dr. Pravin Popatrao Kale,
Department of Pharmacology, SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Gate No. 1, Mithibai College Campus, 1st Floor,
V. L. Mehta Road, Vile Parle (West), Mumbai-400 056, Maharashtra, India.
E-mail: pravinpkale@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Context: Anxiety disorders are chronic, common, and often comorbid. There is an unmet need in its treatment. Aripiprazole and hydroxyzine are well‑known therapeutic options used as monotherapy in clinics. They have different mechanisms and site of actions. Aim: The objective of the present study was to evaluate the anxiolytic effect of aripiprazole and hydroxyzine in combination. Materials and Methods: Swiss albino mice (male) received treatment of 5% of Tween 80 in 0.9% saline (10 ml/kg; control group), “aripiprazole alone” (1 mg/kg), “hydroxyzine alone” (3 mg/kg), and aripiprazole (0.5 mg/kg) + hydroxyzine (1.5 mg/kg) through the intraperitoneal route. Statistical Analysis Used: One‑way ANOVA followed by Tukey’s honest significant difference was employed for statistical analysis. Results: The in vivo outcomes (elevated plus maze, light/dark transition, and marble burying tests) of hydroxyzine monotherapy‑treated group showed a significant anxiolytic activity. The combination‑treated group was found to be better than control and aripiprazole‑treated groups. The combination‑treated group showed a significant increase in the level of serotonin in different brain regions as compared to aripiprazole‑treated group but not better than the hydroxyzine group. The in vitro results were in compliance with the in vivo results. The combinational approach was found to be beneficial in anxiolytic treatment as compared to aripiprazole monotherapy. However, hydroxyzine showed better anxiolytic activity when compared to control, aripiprazole monotherapy, and combination groups. Conclusions: The anxiolytic effect of combination‑treated group was found to be better than aripiprazole monotherapy and lesser than hydroxyzine monotherapy.
Keywords
Anxiety, brain monoamines, cerebral cortex, hippocampus
Introduction
Anxiety disorders lead to a significant distress, cause severe mental and behavioral problems over a period of time. They are characterized by feelings of apprehension, uncertainty, or fear which has a substantial negative impact on the quality of life of an individual. According to the Global Health Estimates 2014, anxiety is ranked sixth in the world as a neuropsychiatric disorder. The prevalence ranges between 2.4% and 29.8%.[1]
Anxiety disorders are highly comorbid and are often associated with substantial impairments in functioning such as depression and attention deficit hyperactivity disorder. Physical symptoms of anxiety include excessive sweating, pounding heart, insomnia, dizziness, headache, fatigue, tremors, and twitches, whereas emotional symptoms include irritability, restlessness, anticipating the worst, inability to concentrate, and feeling tensed.[2] First-line drugs such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are used. SSRIs (paroxetine, citalopram, escitalopram, fluoxetine, sertraline, or fluvoxamine) are generally well tolerated and first-line drugs recommended for patients who never had any drug treatment for anxiety disorders. The success rate of SSRIs is limited up to 61.1%.[3] In addition, SSRIs have been associated with adverse effects such as insomnia, nausea, increased nervousness,[4] and sexual dysfunction.[5] SNRIs such as venlafaxine and duloxetine are alternative first-line agents whereas second-line drugs include tricyclic antidepressants (TCAs) such as imipramine for generalized anxiety disorder and panic disorder clomipramine for obsessive-compulsive disorder and panic disorder and amitriptyline for posttraumatic stress disorder. The success rate of TCAs is limited up to 57.9%.[3] TCAs also have disadvantages such as postural hypotension, weight gain, and insomnia. Alternative second-line drugs are monoamine oxidase inhibitors, namely, phenelzine and tranylcypromine which have side effects such as increased appetite, weight gain, and low blood pressure. Third-line drugs in treatment include benzodiazepines (oxazepam, lorazepam, temazepam, alprazolam, clonazepam, and diazepam), anticonvulsants (carbamazepine, valproate, lamotrigine, topiramate, and gabapentin), atypical antipsychotics, and antihistaminic agents. Benzodiazepines which have lower success rate nowadays are least preferred since they cause physical dependence and withdrawal symptoms.[6] It has been difficult to predict the response rate of the pharmacological therapy of benzodiazepines. There remains uncertainty in deciding an appropriate duration of prescribing these medications.[7] Thus, the use of psychiatric medications for anxiety disorders is being controversial besides the intolerable side effects of available anxiolytic agents and has hindered the desired therapeutic efficacy. This indicates an unmet need in the treatment of anxiety disorders.[8] Combination therapy may help in achieving satisfactory results over monotherapy in combating anxiety disorder.
Aripiprazole is a unique antipsychotic drug. It produces partial agonist action through dopamine D2 receptors and serotonin 5HT1A receptors, whereas antagonist activity through 5HT2A receptors. It behaves as an antagonist in hyperdopaminergic state and an agonist in the hypodopaminergic state.[9] The quality of being a silent antagonist differentiates this drug from other drugs of the same class. It is also known as a dopamine-serotonin system stabilizer. It also possesses anti-aversive properties which would ultimately help in combating anxiety disorder.[10] It is also evident that aripiprazole is well-tolerated, effective, and safe drug with fewer side effects. It is known to produce rapid onset of action with sustained efficacy.[11] Aripiprazole at the dose of 1 mg/kg has shown a significant reduction in the number of marbles buried which represents a defensive behavior in marble burying test.[12]
Hydroxyzine is a widely used antihistaminic, antipsychotic, anxiolytic drug with lesser side effects. It shows its sedative property above the dose of 15 mg/kg in animals.[13] It also possesses tranquilizing activity. It competes with histamine to bind at H1 receptor sites. Hydroxyzine is one of the preferred options in the treatment of a generalized anxiety disorder and psychoneurosis.[14] Hydroxyzine at the doses of 1–4 mg/kg has shown a significant increase in the exploratory activity in light and dark transition test.[15]
Anxiety disorder is influenced by both inhibitory and facilitatory mechanisms which either counter or favor the state of anxiety. The neurochemical systems affect cortical and subcortical brain areas which are pertinent to the mediation of the symptoms associated with anxiety disorders. It is evident that during anxiety, the hippocampus is associated in amplifying the aversive events of anxiety disorder.[16] Changes in dopaminergic and serotonergic systems through hippocampus are related to the anxiety disorders.[17,18] Aripiprazole is categorized as an antipsychotic drug that acts as a partial dopamine agonist and serotonin antagonist. Hydroxyzine belongs to the class of antihistaminic drugs as well as anxiolytic agents that acts as an inverse histamine agonist and serotonin antagonist. The hypothesis of the present study expects synergism from the combination of aripiprazole and hydroxyzine since they have different mechanisms and sites of action. The combination approach consisting half of each monotherapy dose may help in decreasing the dose-related side/adverse effects of each drug while achieving better therapeutic effects. Therefore, the objective of the present study was to evaluate the combined effect of aripiprazole and hydroxyzine on anxiety in mice.
Materials and Methods
Animals
Swiss albino mice (male) weighing 25–30 g were obtained from Bharat Serum Ltd., Thane, India. Six animals were housed in perspex cage. The controlled room temperature (22–24°C) and humidity (50–60%) conditions were maintained at the central animal house facility with a light and dark (12 h: 12 h) illumination cycle. The acclimatization period for animals was 1 week. All experimental protocols were approved by an Institutional Review Committee for the use of animal subjects (Approval number - CPCSEA/IAEC/ BNCP/P-21/2014) and in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication no. 85–23, revised 1985).
Drug solutions and treatment
Animals received the drug treatment through intraperitoneal route. Aripiprazole was received as a gift sample from Watson Pharmaceuticals Pvt. Ltd., Ambernath (District Thane). Hydroxyzine dihydrochloride was procured from Sigma Aldrich, Mumbai, India. Aripiprazole was suspended in Tween 80 (5%) in 0.9% saline.[12] Hydroxyzine dihydrochloride was completely soluble in water.
Experimental protocols were executed between 11.00 and 16.00 h. Each animal model had a separate set of animals. In each set, animals were randomly assigned to four groups (n = 6 animals/group). They were kept undisturbed for at least 1 h before executing experimental protocol. The arena of elevated plus maze (EPM), light and dark transition test, and open field test were wiped with 70% ethyl alcohol solution before placing each animal. Group I, II, III, and IV received treatment of 5% of Tween 80 in 0.9% saline (10 ml/kg; control group), aripiprazole (1 mg/ kg), hydroxyzine (3 mg/kg), and aripiprazole (0.5 mg/kg) + hydroxyzine (1.5 mg/kg), respectively.[12,15,17]
Anxiety models
Elevated plus maze
There was an arrangement of two closed and open arms. The placement of closed arms was opposite to each other (without any roof) with the wall height of 12 cm. Both closed and open arms had dimensions of 30 cm × 5 cm. Each animal was placed in the center of the maze while facing one of the closed arms. A recorded video (5 min) of each animal was used to analyze the total number of entries, the time spent in the open and closed arms.[19] The criterion of an entry was the presence of all four paws inside an arm. The frequency of entries in open arms (OAE) and closed arms (CAE) and total time spent in open arms (OAT) and closed arms (CAT) were evaluated. These data were used for further calculations of percentage of OAT (%OAT) and the percentage of OAE (%OAE).[17]
Light and dark transition test
The dimensions of apparatus (box) were 21 cm × 42 cm with the height of 25 cm. A partition was placed to divide it into two sections of equal size. Dimensions of door in partition wall were 4 cm × 4 cm. The color background of the first compartment was white and the second was black. There was a bright light illumination kept in the first section, while dim in the second section. The mouse was placed in the center place of white box while facing the door present in the partition with the 10 min observation period. Recorded video of each animal was used by a single trained observer to estimate parameters such as time spent in light and dark compartments and percentage time spent in light and dark compartments.[20]
Open field test
The apparatus had a square white-colored wooden arena with the dimensions of 72 cm × 72 cm × 33 cm. The mouse was placed in the center of the arena for 5 min. Recorded video of each animal was used to estimate the parameters such as frequency of line crossing, the time spent in, and entries into the central zone of the arena (18 cm × 18 cm) by a single trained observer.[21]
Novelty suppressed feeding
The dimensions of used wooden box were 72 cm × 72 cm × 33 cm. The arena was covered with a bedding of husk. Animals were food-deprived for 24 h before the commencement of the test. A single pellet of food was placed on a white paper platform positioned in the center of the box at the time of testing. Each mouse was placed at the corner of a novel test box. The recorded video (5 min) of each animal was used to analyze the time taken to approach and eat the chow by a single trained observer. Avoiding food and limited exploration of the test environment represents anxious behavior, whereas quick approach toward food and eating represents a decrease in anxiety.[22]
Marble burying test
The dimensions of plastic cage were 21 cm × 38 cm × 14 cm containing 5 cm thick sawdust bedding. On the sawdust bed, placement of twenty clean glass marbles (diameter = 10 mm approx.) was done evenly. Each animal was placed in a cage for the period of 30 min. After exposure to the marbles, the animal was removed and number of unburied marbles were counted. If 2nd/3rd or higher size of a marble was covered with sawdust bed, then only it was considered buried.[12]
Estimation of monoamine levels (dopamine and serotonin) using high-performance liquid chromatography with fluorescence detector
High-performance liquid chromatography (LC-2010C HT, Autosampler, Shimadzu, Columbia, USA) with fluorescence detector (RF-20A-prominence, Shimadzu, Kyoto, Japan) and a reversed-phase analytical column (KROMASIL 100, C18, 5 μm, 25 mm × 0.46 mm) was used to determine the dopamine and serotonin levels in hippocampus, cerebral cortex, and whole brain (whole brain = cerebral cortex + hippocampus + remaining brain tissue).[23,24]
Euthanasia was performed 1 h after treatment. The brain was removed, weighed, and placed in an ice-cold perchloric acid (0.1 M). Brain parts such as cerebral cortex, hippocampus, and remaining brain tissue were isolated and weighed immediately. Samples were homogenized (Kinematic Polytron PT-MR 2500 E) in 2 ml of ice-cold 0.1 M perchloric acid and the resulting mixture was centrifuged (Eppendorf 5810 R) at 16356 × g for 20 min at the temperature of 4°C. The obtained supernatant was stored at − 80°C after filtration through 0.45 μm membrane up to the time of analysis. The chromatographic separation of injected samples was achieved on reversed-phase analytical column at room temperature. The obtained data were processed by LC Solution@ software (Shimadzu Scientific Instruments 7102 Riverwood Drive Columbia, MD 21046 U.S.A.). The mobile phase was prepared using 0.36 g of potassium orthophosphate, 0.5 ml of phosphoric acid dissolved in 1 liter Millipore water sonicated and filtered through a 0.45 μm membrane (PALL@ Pall Corporation, India). The flow rate of mobile phase was kept at 1.3 ml/min. Detection of dopamine and serotonin was performed at 280 nm excitation wavelength and 315 nm emission wavelength. The retention time of standard and samples was compared and used to identify respective peaks. Analysis of concentration of dopamine and serotonin in the sample was performed by performing their area under curve using their straight line equation. The unit used to express results was μg/g of wet weight of tissue.
Statistical analysis
Statistical analysis was performed using software (GraphPad Software, Inc. 7825 Fay Avenue, Suite 230 La Jolla, CA 92037 USA). The calculation of statistical significance was derived using one-way analysis of variance followed by Tukey’s honest significant difference post hoc test, and the data were represented as a mean ± standard error of the mean values (for each group: n = 6).
Results
Elevated plus maze test
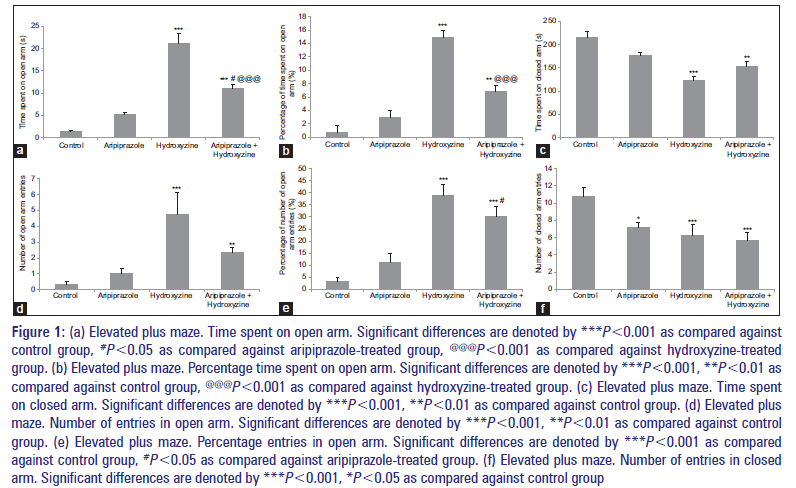
Treatment with aripiprazole resulted in a significant increase in parameters such as OAT, %OAT, OAE, and %OAE, whereas CAT and CAE were decreased when compared against the control group. Similar results were observed with hydroxyzine-treated group when compared against control group. Treatment with the combination of aripiprazole and hydroxyzine resulted in a significant increase in parameters such as OAT, %OAT, OAE, and %OAE, whereas CAT and CAE were decreased when compared separately against control and aripiprazole-treated groups [Figure 1a-f]. Furthermore, the comparison of combined treated group against hydroxyzine-treated group showed a significant decrease in parameters such as OAT, %OAT, OAE, %OAE, and CAE and increase in CAT [Figure 1a-f].
Figure 1: (a) Elevated plus maze. Time spent on open arm. Significant differences are denoted by ***P<0.001 as compared against control group, #P<0.05 as compared against aripiprazole-treated group, @@@P<0.001 as compared against hydroxyzine-treated group. (b) Elevated plus maze. Percentage time spent on open arm. Significant differences are denoted by ***P<0.001, **P<0.01 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (c) Elevated plus maze. Time spent on closed arm. Significant differences are denoted by ***P<0.001, **P<0.01 as compared against control group. (d) Elevated plus maze. Number of entries in open arm. Significant differences are denoted by ***P<0.001, **P<0.01 as compared against control group. (e) Elevated plus maze. Percentage entries in open arm. Significant differences are denoted by ***P<0.001 as compared against control group, #P<0.05 as compared against aripiprazole-treated group. (f) Elevated plus maze. Number of entries in closed arm. Significant differences are denoted by ***P<0.001, *P<0.05 as compared against control group
Light and dark transition test
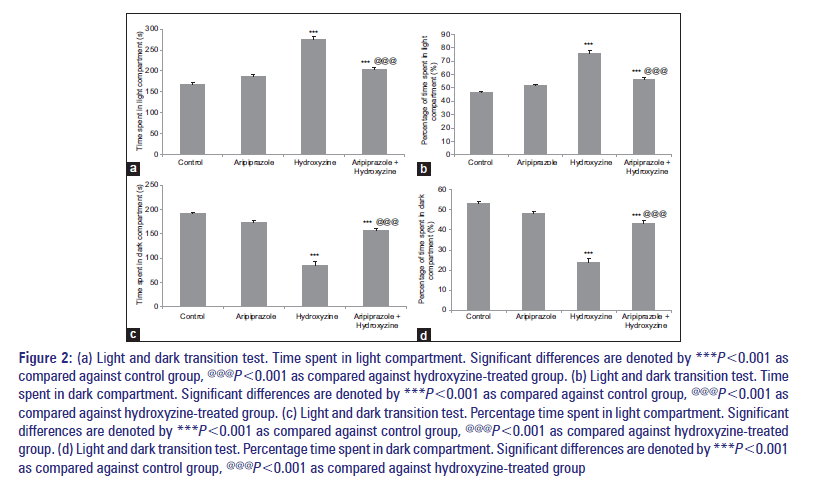
The parameters such as time spent in light compartment and percentage time spent in light compartment were significantly increased with aripiprazole-treated group than the control group. Same group also showed a significant decrease in time spent in dark compartment and percentage time spent in dark compartment than the control group. Similar results were observed with hydroxyzine-treated group when compared against the control group [Figure 2a-d]. Treatment with the combination of aripiprazole and hydroxyzine resulted in a significant increase in time spent in light compartment, percentage time spent in light compartment compared to aripiprazole-treated group. Combination treatment also resulted in decrease in time spent in dark compartment and percentage time spent in dark compartment, as compared to aripiprazole-treated group [Figure 2a-d]. Furthermore, the separate comparison of combination-treated group against hydroxyzine-treated group showed a significant increase in time spent in dark compartment and percentage time spent in dark compartment and decrease in time spent in light compartment, percentage time spent in light compartment [Figure 2a-d].
Figure 2: (a) Light and dark transition test. Time spent in light compartment. Significant differences are denoted by ***P<0.001 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (b) Light and dark transition test. Time spent in dark compartment. Significant differences are denoted by ***P<0.001 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (c) Light and dark transition test. Percentage time spent in light compartment. Significant differences are denoted by ***P<0.001 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (d) Light and dark transition test. Percentage time spent in dark compartment. Significant differences are denoted by ***P<0.001 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group
Open field test
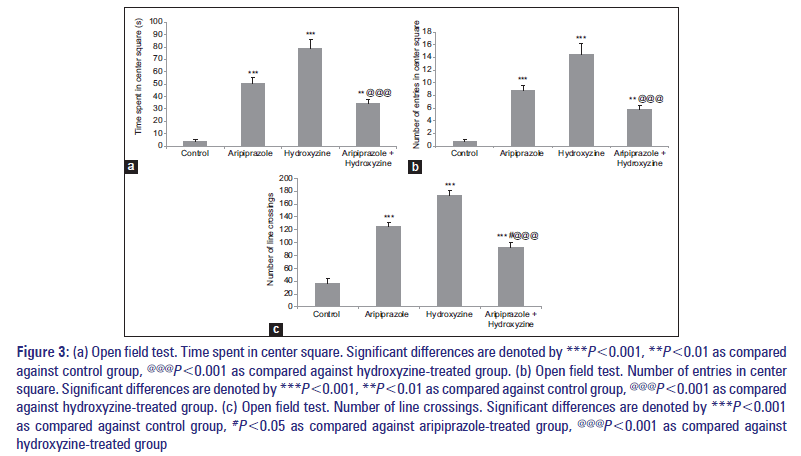
All drug-treated groups which also include combination-treated group showed a significant increase in time spent in center square, center square entries, and line crossing when compared against the control group [Figure 3a-c]. Separate comparison of combination-treated group against aripiprazole- and hydroxyzine-treated groups showed a significant decrease in time spent in center square, center square entries, and line crossings [Figure 3a-c].
Figure 3: (a) Open field test. Time spent in center square. Significant differences are denoted by ***P<0.001, **P<0.01 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (b) Open field test. Number of entries in center square. Significant differences are denoted by ***P<0.001, **P<0.01 as compared against control group, @@@P<0.001 as compared against hydroxyzine-treated group. (c) Open field test. Number of line crossings. Significant differences are denoted by ***P<0.001 as compared against control group, #P<0.05 as compared against aripiprazole-treated group, @@@P<0.001 as compared against hydroxyzine-treated group
Novelty suppressed feeding
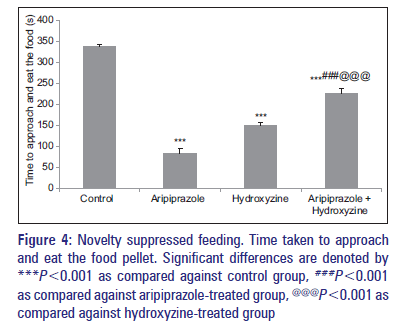
All drug-treated groups which also include combination-treated group showed a significant decrease in the time taken by the mice to approach and eat the food pellet as compared to control group [Figure 4]. Aripiprazole plus hydroxyzine-treated group showed a significant increase in the time taken by the mice to approach and eat the food pellet when compared against aripiprazole- and hydroxyzine-treated groups separately [Figure 4].
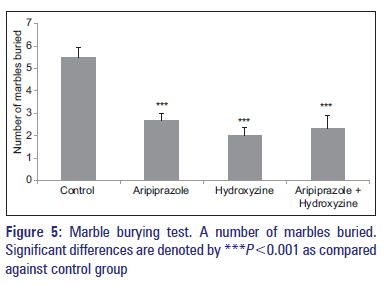
Marble burying test
All drug-treated groups which also include combination-treated group showed a significant decrease in the number of marbles buried by the mice up to two-thirds of the depth when compared against the control group [Figure 5]. Group receiving combination of aripiprazole- and hydroxyzine-treatment showed a significant increase in the number of marbles buried than the hydroxyzine-treated group [Figure 5].
Estimation of brain monoamine (dopamine and serotonin)
Dopamine estimation
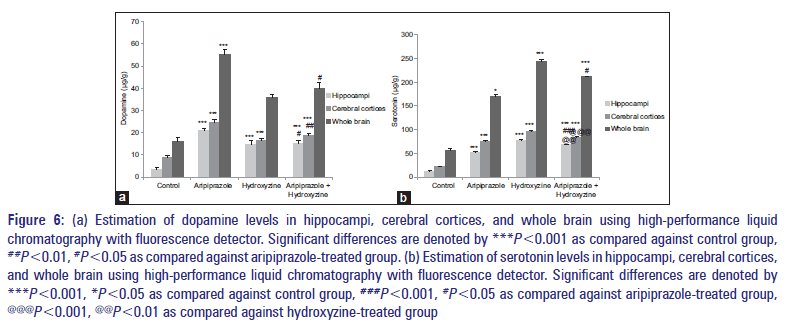
All drug-treated groups which also include combination-treated group showed a significant increase in the levels of dopamine in different brain regions (hippocampi, cerebral cortices, and whole brain) than the control group [Figure 6a]. Comparison of group treated with the combination of aripiprazole (0.5 mg/kg) and hydroxyzine (1.5 mg/kg) against hydroxyzine (3 mg/kg)-treated group showed a significant increase in dopamine levels [Figure 6a].
Figure 6: (a) Estimation of dopamine levels in hippocampi, cerebral cortices, and whole brain using high-performance liquid chromatography with fluorescence detector. Significant differences are denoted by ***P<0.001 as compared against control group, ##P<0.01, #P<0.05 as compared against aripiprazole-treated group. (b) Estimation of serotonin levels in hippocampi, cerebral cortices, and whole brain using high-performance liquid chromatography with fluorescence detector. Significant differences are denoted by ***P<0.001, *P<0.05 as compared against control group, ###P<0.001, #P<0.05 as compared against aripiprazole-treated group, @@@P<0.001, @@P<0.01 as compared against hydroxyzine-treated group
Serotonin estimation
All drug-treated groups which also include combination-treated group showed a significant increase in the levels of serotonin in different brain regions (hippocampi, cerebral cortices, and whole brain) than the control group [Figure 6b]. Group treated with the combination of aripiprazole (0.5 mg/kg) and hydroxyzine (1.5 mg/kg) showed a significant increase in the levels of serotonin in hippocampi, cerebral cortices, and whole brain when compared against “aripiprazole alone” treated group (1 mg/kg) and significant decrease in serotonin levels when compared against “hydroxyzine alone” treated group (3 mg/kg) [Figure 6b].
Discussion
The in vivo results of the present study indicate that combination of aripiprazole and hydroxyzine has better anxiolytic effect as compared to aripiprazole monotherapy. In EPM, the preference of animals of a closed arm (safe and comfortable environment) over open spaces (a risky environment) helps in determining anxiety levels. An increase in time spent and entries in open arm represents anxiolytic behavior.[25] In light and dark transition test, the tendency of animal to explore is reflected by time spent illuminated white compartment and initial tendency to avoid the unfamiliar is reflected by time spent darkened black compartment helps in analyzing anxiety levels.[20] The natural tendency of rodents to spend more time in the corners and the periphery than in the center (the most anxiogenic area) particularly reflecting explore and avoidance reaction to protect itself in the open field test. Qualitative and quantitative measures of general locomotor activity and willingness to explore were the key parameters which reflect anxiolytic behavior.[26] In novelty suppressed feeding test, the ability of an animal to resolve a conflict between a context that induces heightened anxiety and a drive to approach an appetitive stimulus represents anxiolytic behavior.[27] Marble burying test is used to assess the exploratory as well as the defensive behavior of the animal reflecting the state of anxiety and to examine if acute immobilization stress leads to enhanced anxiety-like behavior.[12] The monotherapy of aripiprazole[12,28] and hydroxyzine[15] has shown a considerable anxiolytic effect in different preclinical studies. The present study outcomes of aripiprazole monotherapy[12,28,29] and hydroxyzine monotherapy[17] are in line with the available reports.[12,17,28,29] The combination-treated group showed benefits in terms of exploratory activity in EPM, light and dark transition test, and marble burying test as compared to control group and aripiprazole-treated group, however anxiolytic effect was not better than hydroxyzine treatment in open field test and novelty suppressed feeding test.
It is reported that aripiprazole increases dopamine levels in prefrontal cortex and hippocampus but does not produce significant changes in the levels of serotonin. The aripiprazole-induced elevation in brain monoamines is in line with the available reports.[18] It is evident that H1 receptors play a vital role in anxiety-like behavior.[30] H1 receptor blockers are known to increase the level of serotonin and no effect on dopamine level in the brain.[17] The results of hydroxyzine treatment-induced brain monoamine changes observed in the present study are also in agreement with the in-house finding reports.[17] All drug-treated groups showed benefits in terms of dopamine and serotonin levels in hippocampi, cerebral cortices, and whole brains, as compared to control group. The brain monoamine elevation profile with combination treatment was better than aripiprazole treatment; however, similar benefits were absent when compared against hydroxyzine treatment. The in vitro and in vivo results indicate that combination of aripiprazole and hydroxyzine has better anxiolytic activity than aripiprazole monotherapy only and not against hydroxyzine monotherapy.
The different mechanism of actions and site of action of aripiprazole and hydroxyzine might have helped in achieving improved brain monoamine profile in hippocampi, cerebral cortices, and whole brain than the aripiprazole monotherapy. Aripiprazole belongs to the class of phenylpiperazines. The hepatic enzymes CYP2D6 and CYP3A4 play a vital role in its metabolism. The only known active metabolite of aripiprazole is dehydro-aripiprazole, which typically accumulates to approximately 40% of the aripiprazole concentration.[31] Hydroxyzine also gets metabolized in liver by the CYP2D6 enzyme.[32] Therefore, the chances of drug–drug interaction are high. In addition, the unavailability of related pharmacokinetic reports limits the further understanding of related drug–drug interactions.
Conclusion
The combination of aripiprazole and hydroxyzine at half of their monotherapy doses was beneficial in the treatment of anxiety as compared to aripiprazole monotherapy. However, combination approach was not better than the hydroxyzine monotherapy. The present study findings may assist clinicians in deciding the treatment approach; however, these findings need further assessment in different preclinical or clinical conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol Med 2013;43:897-910.
- Bell-Dolan DJ, Last CG, Strauss CC. Symptoms of anxiety disorders in normal children. J Am Acad Child Adolesc Psychiatry 1990;29:759-65.
- Einarson TR, Arikian SR, Casciano J, Doyle JJ. Comparison of extended-release enlafaxine, selective serotonin reuptake inhibitors, and tricyclic antidepressants in the treatment of depression: A meta-analysis of randomized controlled trials. Clin Ther 1999;21:296-308.
- Baldwin DS, Birtwistle J. The side effect burden associated with drug treatment of panic disorder. J Clin Psychiatry 1998;59 Suppl 8:39-44.
- Segraves RT, Balon R. Antidepressant-induced sexual dysfunction in men. Pharmacol Biochem Behav 2014;121:132-7.
- Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr 2005;10:49-56.
- Baldwin DS. Room for improvement in the pharmacological treatment of anxiety disorders. Curr Pharm Des 2008;14:3482-91.
- Argyropoulos SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drug. Part 2: Pharmacological treatments of anxiety. Pharmacol Ther 2000;88:213-27.
- Greenaway M, Elbe D. Focus on aripiprazole: A review of its use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry 2009;18:250-60.
- Biojone C, Casarotto PC, Resstel LB, Zangrossi H Jr., Guimarães FS, Moreira FA. Anti-aversive effects of the atypical antipsychotic, aripiprazole, in animal models of anxiety. J Psychopharmacol 2011;25:801-7.
- Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90.
- Gaikwad U, Parle M. Combination of aripiprazole and ethanol attenuates marble-burying behavior in mice. Acta Pol Pharm 2011;68:435-40.
- Naghibi B, Rayatnia F. Co-administration of subeffective anxiolytic doses of diazepam and hydroxyzine in elevated zero-maze in mice. Psychiatry Investig 2011;8:169-73.
- Snowman AM, Snyder SH. Cetirizine: Actions on neurotransmitter receptors. J Allergy Clin Immunol 1990;86 (6 Pt 2):1025-8.
- Gladney M, Stanley RT, Hendricks SE. Anxiolytic activity of chloral hydrate and hydroxyzine. Pediatr Dent 1994;16:183-9.
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 2001;21:9896-903.
- Patel S, Kale PP, Addepalli V, Sarkar A, Savai J. Effect of a combination of duloxetine with hydroxyzine on experimental models of anxiety in mice. Indian J Pharmacol 2015;47:173-6.
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett 2005;387:157-61.
- Vogel HG, Vogel WH, editors. Psychotropic and neurotropic activity. In: Drug Discovery and Evaluation, Pharmacological Assays. 3rd ed. Heidelberg: Springer-Verlag; 2008. p. 791.
- Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol 2003;463:55-65.
- Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet 1999;26:263-71.
- Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Repérant C, Orvoën S, et al. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 2013;73:147-59.
- Choudhary KM, Mishra A, Poroikov VV, Goel RK. Ameliorative effect of curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur J Pharmacol 2013;704:33-40.
- Kale PP, Addepalli V. Augmentation of antidepressant effects of duloxetine and bupropion by caffeine in mice. Pharmacol Biochem Behav 2014;124:238-44.
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 2002;134:49-57.
- Kumar V, Bhat ZA, Kumar D. Animal models of anxiety: A comprehensive review. J Pharmacol Toxicol Methods 2013;68:175-83.
- Lo Iacono L, Gross C. Alpha-Ca2/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci 2008;28:6250-7.
- Burda K, Czubak A, Kus K, Nowakowska E, Ratajczak P, Zin J. Influence of aripiprazole on the antidepressant, anxiolytic and cognitive functions of rats. Pharmacol Rep 2011;63:898-907.
- Russo E, Citraro R, Davoli A, Gallelli L, Di Paola ED, De Sarro G.Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology 2013;64:371-9.
- Serafim KR, Kishi MS, Cante-de-Souza A, Morrioli R. H1 but not H2 histamine antagonist receptors mediate anxiety-related behaviours and emotional memory deficit in mice subjected to elevated plus-maze testing. Braz J Med Biol Res 2013;46:440-6.
- Urichuk L, Prior TI, Dursun S, Baker G. Metabolism of atypical antipsychotics: Involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab 2008;9:410-8.
- Hamelin BA, Bouayad A, Drolet B, Gravel A, Turgeon J. In vitro characterization of cytochrome P450 2D6 inhibition by classic histamine H1 receptor antagonists. Drug Metab Dispos 1998;26:536-9.