Evaluation of anti-ulcerogenic properties from the root of Flemingia strobilifera
- *Corresponding Author:
- Anil Kumar KV
Department of Pharmacology, Visveswarapura Institute of Pharmaceutical Sciences, BSK 2nd stage, Bangalore, India
E-mail: anilkumargcp@rediffmail.com
Date of Received :31-05-2010
Date of Modified :03-12-2010
Date of Accepted :22-01-2011
Available Online :15-02-2011
Abstract
PURPOSE: To investigate the anti-ulcerogenic properties of the chloroform extract of Flemingia strobilifera root in rats. METHODS: Anti-ulcer effect was evaluated by water immersion induced ulcer in rats. Other anti-ulcer related activities of the extract such as the effects on free radicals and antimicrobial activity were also evaluated. RESULTS: Chloroform extract of Flemingia strobilifera root was found to be safe up to 300 mg/kg body weight when administrated orally in female wistar rats. Water immer-sion stress produced characteristic lesions in the glandular portion of the rat stomach. Pretreatment with Chloroform extract of Flemingia strobilifera root reduced the char-acteristic lesions in a dose dependent manner (P<0.001) when compared with the control. Pretreatment with Chloroform extract of Flemingia strobilifera root at a dose of 15 and 30 mg/kg body wt. increased the gastric mucosal glutathione level, total protein content significantly (P<0.001) as compared to control group. Whereas there is significant (P<0.05, P<0.001) reduction in gastric mucosal Malonaldehyde levels when compared to control. Free radical scavenging activity of Chloroform extract of Flemingia strobilifera root was observed in the concentration range tested, the IC50 value was calculated. Antimicrobial activity of the Chloroform extract of Flemingia strobilifera root exhibited activity against both gram positive and negative bacteria at concentration of 10 mg/ml. CONCLUSION: The root extract of Flemingia strobilifera possess antiulcerogenic properties could justify folklore uses of the plant in peptic ulcer diseases.
https://marmaris.tours
https://getmarmaristour.com
https://dailytourmarmaris.com
https://marmaristourguide.com
https://marmaris.live
https://marmaris.world
https://marmaris.yachts
Keywords
Flemingia strobilifera root, Antiulcer, Antioxidant, Antimicrobial.
Introduction
Peptic ulcer disease is the term used to describe a heterogeneous group of condition in which there is ulceration of the esophagus, stomach or duodenum. This is apparently associated with some local disturbance of physiological equilibrium. Peptic ulcer disease is one of the most common gastrointestinal disorder, which causes a high rate of morbidity particularly for the population of nonindustrialized countries [1].
Several factors are implicated in the pathogenesis of gastric ulcer including: increased acid-pepsin secretion, impaired bicarbonate neutralization, impaired mucus secretion and precipitate lesions on the mucosal layer [2, 3]. In recent years, a powerful association between peptic ulcers and infection of Helicobacter pylori has been adopted. At least 70-90% of patients with gastric ulcers and 80-95% with duodenal ulcers are infected by H. pylori and eradication of this microorganism seems to be curative for the disease [4]. There is a balance between the aggressive (i.e. acid, pepsin, active oxidants, H. pylori) and the mucosal protective (i.e. mucus, bicarbonate, prostaglandins) factors in the stomach. Thus, drug therapy of peptic ulcer has been commonly targeted at either counteracting the aggressive factors or stimulating defensive ones [5].
The drugs used in the treatment of ulcer include receptor blockers in the gastrointestinal mucus membrane, proton pump inhibitors, drugs affecting the mucosal barrier and those agents which reduce gastric acid secretion mainly by acting on the central nervous system [6]. Even though a range of drugs are available for the treatment of ulcer, many of these do not fulfill all the requirements and have side effects. Despite the progress in conventional chemistry and pharmacology in producing highly effective drugs, some of them are expensive (especially for poor patients) and have different adverse effects [7, 8], however, screening plants for active drugs is still important and might provide a useful source of new anti-ulcer compounds for developing pharmaceutical drugs or alternatively as simple dietary adjuncts to existing therapies [9].
Man has used plants as medicines for thousands of years [10]. The treatment of peptic ulcers with plant products used in folk medicine and the protection of induced gastric ulcer in laboratory animals using medicinal plants was reported [11]. Generally plant flavonoids have been found to be effective against ulcer in experimental animals [12] and exhibit several biological effects.
The genus Flemingia (Fabaceae) comprises over 62 species in the world. In India, this genus is represented by fifteen species, including Flemingia strobilifera [13, 14]. F. strobilifera R.Br, an important medicinal plant, is commonly known as Kusrunt and is found in Sind, Rajputana, Bengal, South India and Andaman’s [15]. Literature reveals that the various parts such as its bracts, leaves, flowers and roots of the plant Flemingia strobilifera found to be useful in folkloric medicine for its different pharmacological activities such as leaves and flower for tuberculosis, roots extracts for ulcers and swellings, roots juice for diarrhea and dysentery [16]. Previous chemical studies showed that flavonoids, flavonoid glycosides, chalcones, epoxychromenes and pterocarpans were the main constituents found in this genus of Flemingia strobilifera R.Br [17, 18]. Hence the present study was undertaken with the aim to assess the antiulcerogenic properties from Flemingia strobilifera claimed by traditional system of medicine.
Experimental
Collection of Plant material
The roots of the plant Flemingia strobilifera R.Br. belonging to family Fabaceae were collected from the Western Ghats of Maharashtra in the month of July 2009. The plant was authenticated by Dr. Jawahar Raveendran, Conservative Research & Action group, FRLHT, and preserved a specimen sample of the same in the herbarium section of the FRLHT, Bangalore-64, with the voucher No. 100154 for future reference.
Preparation of plant extracts
The collected roots were cut in to require size and air dried then extracted with chloroform at 50ºC and the extract so obtained was filtered. The procedure was again repeated five times using adequate amount of chloroform at an interval of 3 days. The filtrate was evaporated to dryness to get residue. Then the residue was transferred to a china dish and evaporated on thermostat controlled water bath at 40ºC and stored in a refrigerator until further use. The amount of extract collected was 50 gm w/w from the dried powdered root of Flemingia strobilifera R.Br.
Animals
Albino wistar rats (150-200g) of either sex used for the study were obtained from Drug control laboratory and Institution of veterinary science Bangalore, Karnataka. After one week of acclimatization the animals were used for experiments. The protocol for the study was approved by institutional animal Ethics committee (Reg. no.152/1999/CPCSEA) as per the Indian CPCSEA guidelines.
Acute Toxicity Studies
The acute toxicity was determined on virgin female albino wistar rats by fixed dose method of OECD Guide line no 420 given by CPCSEA [19]. Groups of 6 rats were administered test drug by oral route at a dose of 2000, 300mg/kg (6 animals in each dose) [19] and mortality was observed after 24 hr. The safe dose was found to be 300 mg/kg body weight. For the study two doses were selected, 30 mg/kg body weight and 15 mg/kg body weight (1/10th, 1/20th of the maximum safe dose).
Water immersion stress induced ulcer in rats
Stress ulcers were induced by forcing the Wistar albino rats of either sex to swim in the glass cylinder [20] containing water to the height of 35 cm maintained at 25ºC for 3 h. The animals were divided into four groups each group consist of six animals. They were fasted for 24 h prior to the experiment. Group I received 1.0 ml/200gm p.o. 10% tween 80 as vehicle (control), Group II received 25 mg/kg, p.o. Standard used was Ranitidine. Group III and IV respectively received 30 mg and 15 mg/kg, p.o. chloroform extract of the root Flemingia strobilifera. After 30 min of the respective treatment animals were allowed to swim for 3 hrs. Then they were killed with high dose of anaesthetic ether. The stomach of each animal was cut longitudinally along the greater curvature and the stomach were washed with normal saline and severity of the ulcers were scored (i.e.0= normal colored stomach, 0.5=red colouration, 1=spot ulcer, 1.5=hemorrhagic streaks, 2=ulcers>3mm but<5mm, 3=ulcers>5mm) [21]. The other parameters such as percentage protection [22], reduced Glutathione level [23], Lipid per oxidation [24] and Total protein content [25] were evaluated in the stomach tissue.
In vitro antioxidant studies
Free radical scavenging potentials of the extract were tested against a methanolic solution of 1, 1-diphenyl- 2-picryl hydrazyl (DPPH). Th is spectrophotometric assay uses the stable radical DPPH as a reagent. 1 ml of various concentrations of the extract in methanol was added to 4 ml of 0.004% methanol solution of DPPH. Gallic acid (Sigma, USA) was used as a standard free radical scavenger (50 mg of Gallic acid was dissolved in 100 mL of methanol). After 30 min of incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition of free radical by DPPH in percent (I %) was calculated in following way:
I (%) = (A blank – A sample / A blank) x 100
where,
A blank is the absorbance of the control reaction (containing all reagents except the test compound) A sample is the absorbance of the test compound.
Extract concentration providing 50% inhibition (IC50) was calculated from the plot of inhibition (%) against extract concentration. Tests were carried out in triplicate [26].
In vitro-antimicrobial activity studies
Helicobacter pylori, the enteric organism implicated in peptic ulcer disease was not used in this screening test because of the difficulty in culturing the organism. However, other entheropathogenic and related gram positive and gram negative microorganisms were employed. The organisms used were: Escherichia coli and Staphylococcus aureus [27]. They were clinical strains obtained from the KIMS (Kempegowda Institute of Medical Sciences) Microbiology Laboratory, Bangalore. The organisms were maintained by weekly sub culturing and incubating at 37ºC. 24 h old cultures of the microorganisms were used in the screening. The agar disc diffusion method was employed. Wells of 8 mm diameter were bored on seeded gelled agar dish containing 1.0 x 106 cfu /ml of the respective organism and varying concentrations (5-10 mg/ml) of the extracts applied to the appropriately labeled wells. The plates were incubated at 37ºC for 24 h. The effects of the extract on the growth of the microorganisms were studied by observing the zones of inhibitions. The experiments were carried out in triplicates and the mean inhibition zone diameter was observed in each case.
Analysis of results
The results were expressed as mean ± SEM and were analyzed for statistically significant difference using one-way ANOVA followed by Tukey’s Kramer post hock test. P values < 0.05 were considered significant.
Results
Chloroform extract of Chloroform extract of Flemingia strobilifera root was found to be safe up to 300 mg/kg body weight when administrated orally in female Wistar rats.
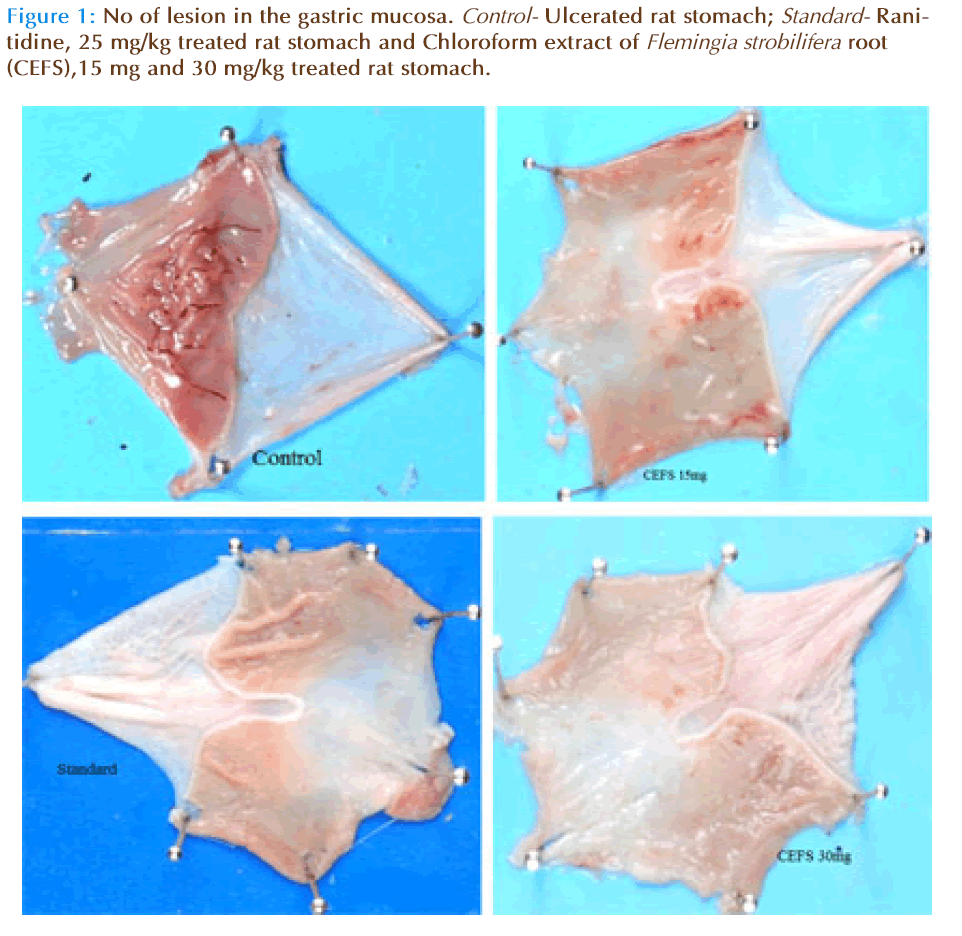
Water immersion stress produced characteristic lesions in the glandular portion of the rat stomach. Pretreatment with Chloroform extract of Flemingia strobilifera root reduced the characteristic lesions in a dose dependent manner as shown in the figure 1. Table 1 shows that Chloroform extract of Flemingia strobilifera root produced dose dependent (P<0.001) protection of gastric mucosal ulceration when compared with the control. Pretreatment with CEFS at a dose of 15 and 30 mg/kg body wt. increased the gastric mucosal GSH level, total protein content significantly (P<0.001) as compared to control group.
| Treatment | Dose (mg/kg) | Ulcer index | GSH(μmol/100mg) | Total protein(μg/100mg) | MDA (μmol/gm) |
|---|---|---|---|---|---|
| Control | 1ml/100g. | 7.91 ± 0.611 | 1.984 ± 0.103 | 2.98 ± 0.339 | 4.45 ± 0.114 |
| Standard | 25 | 1.98 ± 0.568*** | 3.336 ± 0.294** | 5.81 ± 0.357** | 3.45 ± 0.260* |
| CEFS | 15 | 4.93 ± 0.307** | 2.963 ± 0.204* | 3.38 ± 0.241* | 4.03 ± 0.139ns |
| CEFS | 30 | 3.25 ± 0.559*** | 3.47 ± 0.249*** | 5.43 ± 0.355** | 3.32 ± 0.285** |
Values expressed as mean ±SEM, n=6, ANOVA followed by Tukey’s Kramer post hock test, *p<0.05, ** p<0.01, *** p<0.001 when compared with control.
Table 1: Effect of Chloroform extract of Flemingia strobilifera root (CEFS) against Water immersion induced ulcer
Whereas there is significant (P<0.05, P<0.001) reduction in gastric mucosal MDA (Malonaldehyde) levels in the animals pretreated with 15 and 30 mg/ kg body wt. of Chloroform extract of Flemingia strobilifera root when compared to control. Standard drug treated group also showed significant activity as compared to control group.
DPPH has been used extensively as a free radical to evaluate free radical scavenging activity. Activity of Chloroform extract of Flemingia strobilifera root was observed in the concentration range tested, the IC50 value was found to be 193.38 μgm/ml. %, which was the lowest antioxidant performance. In this study gallic acid used as a reference standard and its IC50 value was found to be 2.7μgm/ml (Table 2).
| Sample | Concentration tested (μg/ml) | % inhibition | IC50 |
|---|---|---|---|
| 1 | 36.36 | ||
| Gallic acid | 5 | 54.42 | 2.7μg/ml |
| 10 | 71.53 | ||
| 50 | 31.39 | ||
| CEFS (Root extract) | 100 | 39.27 | 193.38 μg/ml |
| 200 | 50.55 | ||
| 250 | 54.34 |
Table 2: IC50 data of the tested sample in DPPH free radical scavenging assay
Antimicrobial activity recorded in terms of average zones of inhibition in millimeter (mm) was shown in Table 3. The Chloroform extract of Flemingia strobilifera root showed a range of activity against Staphylococcus aureus (gram positive) and E. coli (gram negative). The Chloroform extract of Flemingia strobilifera root exhibited activity against both gram positive and negative bacteria at concentration of 10 mg/ml. The standard drug Streptomycin showed strong activity against these organisms.
| Microorganism | CEFS root, conc.(mg/ml) | Zone of inhibition (mm) |
|---|---|---|
| Staphylococcus aureus | 5 mg/ml | _ |
| 10 mg/ml | + | |
| E. coli | 5 mg/ml | _ |
| 10mg/ml | + | |
- = No inhibition.
+ = ≤5 mm diameter of zone of inhibition.
Table 3: Antimicrobial activity of Chloroform extract of Flemingia strobilifera root (CEFS)
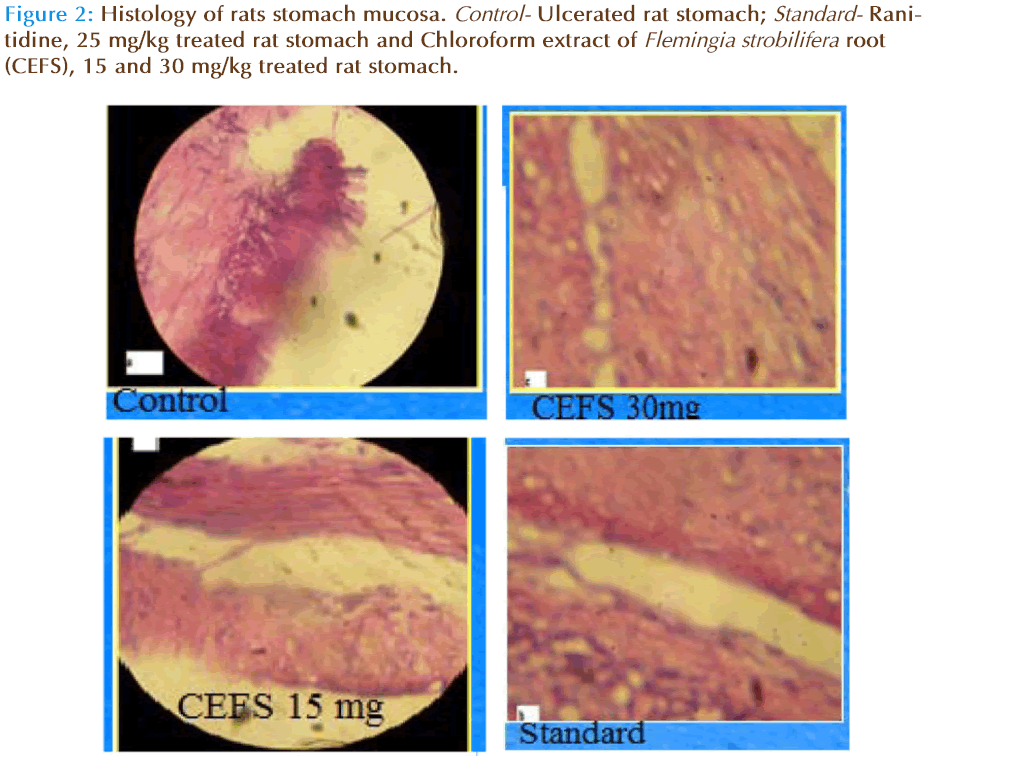
The histopathology of gastric mucosa of rats reveals a significant reduction in gastric erosion and lesions in Chloroform extract of Flemingia strobilifera root treated group, which is similar to that in the Ranitidine treated group as compared to the control group (Figure 2).
Discussion
The present study indicate that Chloroform extract of Flemingia strobilifera root showed antiulcerogenic properties against water immersion induced ulcer model and also it proved to have antioxidant and antimicrobial activity. The extract conferred some degree of protection against ulcers induced by water immersion in rats. Water immersion induced ulcer model is associated with increase in gastric acid secretion and a decrease in pH causes digestion of mucus membrane, forms a characteristic lesion on the stomach mucosa. Histamine is believed to have an essential role in the pathogenesis of stress-induced ulcer since it is a potent stimulator of gastric acid secretion [28].
Peptic ulcer and gastritis have multietiopathogenetic factors. It is widely accepted that a major under lying factor of this disorder is the generation of free radicals. There is substantial evidence that oxygen derived free radicals play an important role in the pathogenesis of the injury of various tissues, including the digestive system [29, 30]. In addition, involvement of oxygen derived free radicals such as the superoxide anion, hydrogen peroxide, and hydroxyl radical are well established in the pathogenesis of ischemic injury of gastrointestinal mucosa and in other pathogens of mucosal damage induced by water immersion and H.pylori [29]. Lipid peroxidation product of MDA is thought to refl ect free radical mediated cell membrane damage. It is known that radical scavengers, such as alfa tocopherol, carotenoids and glutathione redox system, play a significant role in protecting membranes from oxidative damage. Depletion of gastric mucosal GSH may result in the accumulation of free radicals that can initiate membrane damage by lipid peroxidation. Salim et al. investigated the infl uence of free radical scavengers on the healing of gastric and duodenal ulcers resistant to therapy and found that antioxidative therapy stimulates the healing of therapy resistant ulcers [31]. In our present study the decreased glutathione levels were increased upon Chloroform extract of Flemingia strobilifera root treatment, indicating an enhanced antioxidant status, reduced lipid peroxidation with enhanced ulcer protection.
Microbial colonization of the gastrointestinal system has been associated with a variety of peptic ulcer diseases. Helicobacter pylori, the enteric organism implicated in peptic ulcer disease were not used in this screening test because of the difficulty in culturing the organism. However, other entheropathogenic and related microorganisms were employed. The organisms used were Escherichia coli, and Staphylococcus aureus. In in-vitro antimicrobial activity, Chloroform extract of Flemingia strobilifera root (5- 10mg/ml) inhibited the growth of gram positive and gram negative micro organism.
In addition, the antiulcer and antimicrobial activities of the Chloroform extract of Flemingia strobilifera root may be attributed to its fl avonoid content. Th is postulation is consistent with the earlier observations that fl avonoids possess antibacterial, spasmolytic, antiulcerogenic [32] and antigastric activities as well as ability to inhibit acid secretion [33]. Most of these effects have been attributed to the infl uence of fl avonoids on arachidonic acid metabolism, their vasoprotective action [34] and their ability to interfere with the formation of histamine in the gastric mucosa [28].
Conclusion
The chloroform extract of Flemingia strobilifera R.Br. root at 15 mg and 30 mg/kg, body wt. p.o. exhibited significant anti-ulcerogenic properties evidence by physical, biochemical and Histopathological parameter. Further study need to be done to elucidate the mechanism of action involved in the antiulcer activity.
References
- Falk GW. Disease of the stomach and duodenum. In: Andreoli TE, editor. Cecil essentials of medicine. 5th ed. Edinburgh: W.B. Saunders Company; 2001.
- Kent-Lioyd KC, Debas HT. Peripheral regulation of gastric acid secretion. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1994.
- Glavin GB, Szabo S. Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J 1992; 6: 825-831.
- McQuaid KR. Alimentary system. In: Tierney LM, McPhee SJ, Papadakis MA, editors. Current medical diagnosis and treatment. 41st ed. New York: Lange Medical Books/McGraw Hill Company; 2002.
- Tepperman BL, Whittle BJR. Endogenous nitric oxide and sensory neuropeptides interact in the modulation of the rat gastric microcirculation. Br J Pharmacol 1992; 105: 171-5.
- Manonmani S, Viswanathan VP, Subramanian S. Biochemical studies on the antiulcerogenic activity of cauvery 100, an ayurvedic formulation in experimental ulcers. Ind. J Pharmacol 1995; 27: 101-105.
- Anoop A, Jegadeesan M. Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus R. Br. Var. indicus. J Ethnopharmacol 2003; 84: 149-156.
- Dharmani P, Mishra PK, Maurya R. Allophylus serratus: A plant with potential anti-ulcerogenic activity. J Ethnopharmacol 2005; 99: 361- 366.
- Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res 2000; 14: 581-591.
- Suffredini IB, Bacchia EM, Sertie JAAA. J Ethnopharmacol 1999; 65: 217.
- Ahmad MD, Salah O, Ghaleb M, et al. J Ethnopharmacol 1998; 60: 189.
- David A, Lewis, William N, Fields. Shaw J Ethnopharmacol 1999; 65: 283.
- Chopra RN, Nayer SL, Chopra IC. Glossary of Indian medicinal plants. C.S.I.R Publication; 1965 .p. 220.
- Kirtikar KR, Basu BD. Indian medicinal plants. Lacit mohan basu M.B 49, Leader road. Allahabad; 1935.
- Duthic JS. Flora of upper gangetic plain. Bishen sing Mohendra pal singh new connerght place. Dheradoon; 1994.
- Luis A, Jaime A, Guilermo SH. Gastro protective activity of oleanolic acid derivatives on experimentally induced gastric lesion in rats and mice. Journal of Pharmacy and Pharmacol 2002; 54: 583.
- Madan S, Singh GN, Kumar Y. A New Flavanone from Flemingia strobilifera (Linn) R.Br. and its Antimicrobial Activity. Trop J Pharm Res 2008 Mar ; 7 (1): 921-27.
- Madan S, Gyanendra NS, Kanchan K. Isofl avonoids from Flemingia strobilifera (L) R.Br. roots. Acta Poloniae Pharmaceutica-Drug research 2009; 66(3): 297-303.
- OECD. Guidance document on acute oral toxicity. Environmental Health and safety monograph series on testing and assessment 2000; 24.
- Bhattacharya SK, Bhattacharya D. Effect of restraint stress on rat brain serotonin. Journal of Bioscience 1982; 4: 269–274.
- Kulkarni SK. Hand book of Experimental Pharmacology. Vallabh prakashan, New Delhi; 1987.
- Hawk PB, Oser BL and Summerson HW. Practical physiology chemist 12th edition. Churchill London; 1947: 347
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959; 82: 70-7.
- Ohkawa H, Onishi N, Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-58.
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the folin- phenol reagent. J Biol Chem 1951; 193: 265-275.
- Bandyopadhyay U, Das D, Banerjee KR. Reactive oxygen species: Oxidative damage and pathogensis. Curr Sci 1999; 77: 658-66.
- Makinde1 AA, Igoliz JO, Ama1 LTA. Antimicrobial activity of Cassia alata. African Journal of Biotechnology 2007July 4; 6 (13): 1509-1510.
- Ibu JO. Hypoglycaemic action of gastrin. Biologia Africana 1985; 2(2): 22-27
- Santra A, Chowdhury A, Chaudhuri S. Oxidative stress in gastric mucosa in helicobacter pylori infection. Indian J Gastroenterol 2000; 19: 21-3.
- Choi MA, Kim BS, Yu R. Serum antioxidative vitamin levels and lipid peroxidation in gastric carcinoma patients. Cancer Lett 1999; 136: 89-93.
- Kusumoto IT, Nakabayashi T, Kida H. Screening of various plant extracts used in Ayurvedic medicine for inhibitory effects on human immunodeficiency virus type I (HIV-I) protease. Phytotherapy Research 1995; 12: 488–493.
- Carlo GD, Mascolo N, Izzo AA. Effects of quercetin on the gastrointestinal tract of rats and mice. Phytother Res 1994; 8: 42 – 45.
- Das P. Flavonoids in Biology and Medicine 111. National University of Singapore for Biochem Pharmac 1986; 32: 1141 – 1148.
- Parmer NS, Ghoshi MN. Antigastric activities of bifl avonoids in rats. Tavahabal Inst Post Grad Med Educ Res 1976; 1: 6.