Evaluating sanitization of toothbrushes using ultra violet rays and 0.2% chlorhexidine solution: A comparative clinical study
- *Corresponding Author:
- Dr. Poonam Tomar
Department of Public Health Dentistry, People’s Dental Academy, People’s University, Bhanpur, Bhopal, Madhya Pradesh, India.
E-mail: dr.poonamtomar1@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Toothbrushes may play a significant role in plaque control. Toothbrushes should be correctly stored, disinfected and changed at regular intervals. Objective: The purpose of this study was to evaluate the efficacy of 0.2% chlorhexidine (CHX) gluconate solution and ultra violet (UV) toothbrush‑sanitizer for toothbrush disinfection. Materials and Methods: Fresh tooth brushes were distributed to fifteen study subjects, who were selected randomly and who met the study criteria. All the study participants were asked to brush their teeth with the tooth brush provided. No special instructions were given regarding the brushing techniques. Toothbrushes were collected after 7 days. All tooth brushes were randomly allocated to three groups. Tooth brushes were subjected to microbial analysis and total bacterial count was assessed. Tooth brushes allocated to Group I were soaked in 2% CHX mouthwash for 12 h, Group II were kept in UV‑light toothbrush holder for 7 min, and Group III were soaked in normal saline for 12 h. All the toothbrushes were subjected for microbial analysis and mean bacterial count was determined. Results: There was a statistically significant difference between mean colony‑forming unit count pre‑sanitization and post‑sanitization in all the groups, using 0.2% CHX gluconate, UV rays and normal saline (P < 0.007). However, the mean bacterial count reduced drastically after the treatment with UV rays (P = 0.001). Conclusions: CHX, UV rays and normal saline are effective in a reduction of bacterial count on toothbrushes. UV rays treatment was more effective, when compared to CHX and normal saline.
Keywords
Chlorhexidine gluconate, disinfectant, toothbrush contamination, ultra violet radiation
Introduction
The most common oral hygiene aid used to improve the oral health of an individual is toothbrush. Tooth brushing plays an important role in personal oral hygiene and plaque control. Toothbrushes may become heavily contaminated with microorganisms from the oral cavity, environment, hands, aerosol contamination, and storage containers. [1] Micro-organisms that attaches, accumulate, and survive on toothbrushes may be transmitted to the individual, which in turn can further cause diseases. [2]
After a single use for duration ranging from 30 s to 4 min, however, toothbrushes may become contaminated by a wide array of bacteria, viruses, yeasts, and fungi, which are present both in the oral cavity and in the external environment. [3]
Prolonged use of the toothbrush facilitates contamination by various micro-organisms such as Streptococcus, Staphylococcus, and Lactobacilli. These micro-organisms are implicated to cause dental caries, gingivitis, stomatitis and even infective endocarditis in an individual, affecting both oral and general health. [4] Microorganisms can remain viable on toothbrush bristles for periods ranging from 24 h to 7 days. [5]
Cobb (1920) reported that the toothbrush to be a cause of repeated infections of the mouth. [6] Toothbrushes may play a significant role in disease transmission, and increase the risk of infection since they can serve as a reservoir for microorganisms in healthy, medically ill and adults with poor oral health. [2] Unfortunately, proper care of toothbrush is often neglected and is kept in bathrooms that are a good place to harbor millions of micro-organisms. [4]
Svanberg found that toothbrushes can be heavily infected by mutans streptococci after 24 h. Mutans streptococci cells exist in moist dental plaque that adheres to and can remain on toothbrushes; several studies have focused on toothbrush disinfection methods so far. [7]
Procedures for the decontamination of toothbrushes would prevent the risks of reinfection or infection by other pathogenic microorganisms from the environment. [1] Over the years, numerous methods of toothbrush sanitization have been put forward, such as exposure to ultraviolet light and microwaves, disinfectant tablets, and immersion in solutions such as Clorox and antimicrobial agents. [8]
Among the chemical agents, chlorhexidine (CHX) gluconate solutions (0.12%) have proved efficient toothbrush disinfection. It has been reported in successfully eliminating microbial species such as Streptococcus mutans, Candida albicans, Staphylococcus aureus and Streptococcus pyogenes within 10 min. [9]
Although various sanitization techniques that have been tried exhibited varying levels of effectiveness, none of them proved to be the ideal sanitization method for decontamination of tooth brushes. [8]
Modern dentistry strongly emphasizes prevention and bio-security regarding how toothbrushes should be appropriately stored, disinfected, and changed at regular intervals.
Concerns regarding instruments for oral cleaning such as toothbrushes and dental floss have always existed. Although methods for tooth brushing are described in the literature, procedures for maintaining the cleanliness of toothbrushes are rarely discussed. [10]
Therefore, the present study is under taken to evaluate the efficacy of 0.2% CHX gluconate solution, ultra violet (UV) toothbrush-sanitizer and normal saline for toothbrush disinfection.
Materials and Methods
This short term clinical trial was conducted for the duration of 1 week among a group of 15 adults with age range of 21–50 years old, working in a private institution. The study evaluated the sanitization of toothbrushes using UV rays, 0.2% CHX gluconate solution and normal saline. Ethical clearance was obtained from the institution ethics committee. Informed consent was also obtained from the participants after explaining the entire research protocol.
Source of data
The study subjects consisted of 15 adults, who fulfilled the necessary inclusion criteria. Further, only those individual who provided written informed consent were included.
Method of collection of data
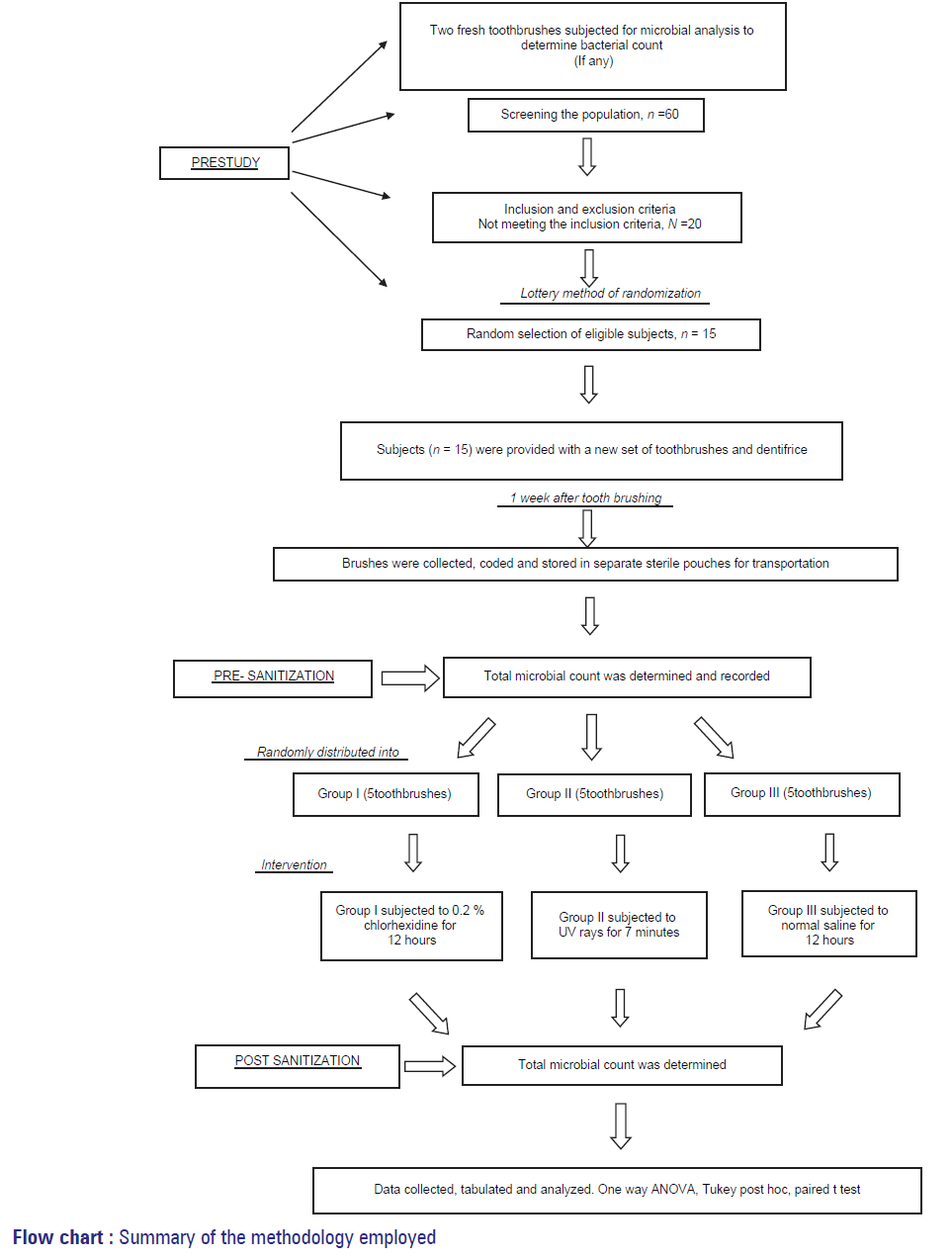
The entire study was conducted in three phases, broadly categorized as pre study phase, intervention phase and post-sanitization phase (flowchart 1).
Prestudy
A checklist was designed for collecting the information on demographic details, oral hygiene practices, medical history, and details of the oral examination, etc., This check list facilitated in making necessary exclusions and inclusions for the study. The checklist consisted of 11 multiple questions.
Of the total 60 subjects, 15 individuals fulfilled the inclusion criteria (subjects good in general health, having at least 20 natural teeth per arch, those who were able to give written informed consent and complied with the study protocol.) and were included in the study. Those individuals who failed to give the written informed consent were excluded from the study.
All the study participants were provided with an oral hygiene kit containing a toothbrush and a dentifrice. To standardize and maintain the uniformity with regards to mechanical plaque control, the dispensed toothbrushes were soft bristled with uniform make. Furthermore, the fluoridated dentifrice (80 g each) dispensed in the kit, served the dual purpose of increase the compliance of study participants and maintaining the uniformity regarding plaque control measures.
The study groups were asked to refrain from all other unassigned forms of oral hygiene practices. All the participant were instructed to brush once daily (in the morning using about a gram of toothpaste) for a period of 2-4 min with the assigned toothpaste for a period of 1 week. Following the brushing, they were asked to rinse their toothbrush in running tap water for 30 s and thereafter place the brush in an open brush holder, bristles up, outside the bathroom. After 1 week, all the used toothbrushes were collected by the investigator and stored in sterile plastic pouches. All the tooth brushes were coded from 1 to 15 numbers. The tooth brushes were promptly delivered to the laboratory for bacterial extraction. Prior to the study, two fresh tooth brushes identical to the brushes used in the study were subjected to microbial analyses to determine the total bacterial count if any.
Intervention using different sanitization procedure
The toothbrushes were numbered from 1 to 15, and total viable bacterial count was determined and analyzed for the number of colony-forming units (CFUs).All the toothbrushes were randomly allocated into three test groups.
Group I: Five toothbrushes soaked in 0.2% CHX mouthwash for 12 h
Group II: Five toothbrushes placed in ultraviolet chamber for 7 min (portable duo toothbrush sterilizer)
Group III: Five toothbrushes in normal saline for 12 h.
Following the intervention in the respective groups, all the toothbrushes were again subjected for the microbial analysis to determine the efficacy of various sanitization procedures.
Post-sanitization evaluation
The handle of each toothbrush was disinfected with a surgical spirit. The tooth brushes were individually placed in sterile test-tubes, containing 0.9% sodium chloride (NaCl2) solution. The bristles were immersed in normal saline and vortexed vigorously for 5 min. A serial dilution of 10−1 up to 10−12 for each sample was prepared. The diluted solutions were uniformly dispensed on nutrient agar media by pour plate technique. These petri plates were then incubated for 24 h at 37°C to facilitate the microbial colony formation. The number of colonies, measured as CFU’s was counted using a digital colony counter.
Data analysis
The data obtained for all the microbial counts were entered onto a personal computer and statistical analysis was performed using SPSS version 20 (IBM, Chicago, USA). The total bacterial count (CFU) after tooth brushing contamination and sanitization (decontamination) were compared and analyzed using one-way ANOVA. Tukey’s post-hoc was used for multiple comparisons. Paired t-test was used to compare microbial count before and after the intervention. The statistical significance was fixed at 0.05.
Results
The study was conducted among 15 participants, who consented and fulfilled the inclusion and exclusion criteria, and were considered for clinical oral examination. Prior to the intervention, the range and mean CFU count was assessed by microbial analysis. Following the intervention in the respective groups, all the toothbrushes were again subjected for the microbial analysis to determine the efficacy of various sanitization procedures.
Comparison of the pre disinfection microbial contamination between different groups
A series of dilution from 10−1 to 10−9 was prepared from the stock solution. Significant results obtained at dilutions 10−6, 10−7, 10−8 were recorded. The range at different dilutions was 123–321 CFU. The mean ± SD CFU counts observed in Group I (CHX) was 295 ± 28.879 at 10−6 dilution, 272.20 ± 30.111 at 10−7 dilution and 237.60 ± 32.982 at 10−8 dilution. The mean ± SD observed in Group II (UV Rays) was 300.60 ± 8.678 at 10−6 dilution, 264.00 ± 21.7600 at 10−7 dilution and 223 ± 29.223 at 10−8 dilution. The mean ± SD observed in Group III (Saline) was 265.80 ± 39.264 at 10−6 dilution, 224.60 ± 43.718 at 10−7 dilution and 189.20 ± 44.802 at 10−8 dilution.
There was no statistically significant difference among the three groups at various dilutions (P > 0.05) [Table 1].
| Presanitization | ||||||
|---|---|---|---|---|---|---|
| Groups | CFU at 10−6 dilution | CFU at 10−7 dilution | CFU at 10−8 dilution | |||
| Range | Mean±SD | Range | Mean±SD | Range | Mean±SD | |
| Group I (chlorhexidine) | 246-321 | 295.00±28.879 | 225-300 | 272.20±30.111 | 191-273 | 237.60±32.982 |
| Group II (UV rays) | 288-311 | 300.60±8.678 | 231-286 | 264.00±21.760 | 189-266 | 223.00±29.223 |
| Group III (saline) | 202-307 | 265.80±39.264 | 160-271 | 224.60±43.718 | 123-236 | 189.20±44.802 |
| F=2.137 | F=2.951 | F=2.341 | ||||
| P=0.161 (NS) | P=0.091(NS) | P=0.139 (NS) | ||||
NS: Not significant, UV: Ultra violet, SD: Standard deviation, CFU: Colony forming unit
Table 1: Comparison of mean microbial CFUs before intervention at different serial dilutions
Comparison of the post-disinfection microbial contamination between different groups
The range of CFU counts at different dilutions is 15–202. The mean ± SD observed in Group I (CHX) was 183.40 ± 8.112 at 10−6 dilution, 138.80 ± 12.755 at 10−7 dilution and 87 ± 4.950 at 10−8 dilution. The mean ± SD observed in Group II (UV Rays) was 46.60 ± 10.807 at 10−6 dilution, 24.60 ± 6.731 at 10−7 dilution and 18 ± 2.236 at 10−8 dilution. The mean ± SD observed in Group III (Saline) was 163 ± 25.377 at 10−6 dilution, 134 ± 31.089 at 10−7 dilution and 95.40 ± 7.162 at 10−8 dilution. There was a statistically significant difference among all the three groups after intervention at various dilutions (P = 0.001) [Table 2].
| Groups | CFU at 10−6 dilution | CFU at 10−7 dilution | CFU at 10−8 dilution | |||
|---|---|---|---|---|---|---|
| Range | Mean±SD | Range | Mean±SD | Range | Mean±SD | |
| Group I (chlorhexidine) | 174-194 | 183.40±8.112 | 126-160 | 138.80±12.755 | 81-93 | 87±4.950 |
| Group II (UV rays) | 35-60 | 46.60±10.807 | 18-35 | 24.60±6.731 | 15-21 | 18±2.236 |
| Group III (saline) | 138-202 | 163±25.377 | 103-178 | 134±31.089 | 89-102 | 95.40±7.162 |
| F=98.837 | F=53.284 | F=334.849 | ||||
| P=0.001 (S) | P=0.001 (S) | P=0.001 (S) | ||||
S: Significant, UV: Ultra violet, SD: Standard deviation, CFU: Colony forming unit
Table 2: Comparison of mean microbial CFUs after intervention at different serial dilutions
Comparison of mean microbial colony-forming units before and after intervention (pre- and post-sanitization)
A reduction in the total viable count following sanitization compared to baseline levels was noted with all the three disinfectants. The reduction in the total viable count following sanitization compared to baseline levels was noted in all the three groups even at 10−7 and 10−8 serial dilution. The comparison of pre-sanitization CFU values with the post-sanitization CFU count reveals that all the three methods were effective in reducing the microbial load. There was statistically significant difference between pre-sanitization and post-sanitization CFU count (P < 0.007) [Table 3].
| Dilution | Group I chlorhexidine | Group II UV rays | Group III saline | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | P | Mean±SD | P | Mean±SD | P | ||||
| Pre | Post | Pre | Post | Pre | Post | ||||
| 10−6 | 295±28.879 | 183.40±8.112 | t=8.032 | 300.60±8.678 | 46.60±10.807 | t=55.422 | 265.80±39.264 | 163±25.377 | t=9.438 |
| P=0.001 | P=0.001 | P=0.001 | |||||||
| 10−7 | 272.20±30.111 | 138.80±12.755 | t=8.234 | 264.00±21.7600 | 24.60±6.731 | t=26.158 | 224.60±43.718 | 134±31.089 | t=7.316 |
| z=0.001 | P=0.001 | P=0.002 | |||||||
| 10−8 | 237.60±32.982 | 87±4.950 | t=11.029 | 223±29.223 | 18±2.236 | t=15.617 | 189.20±44.802 | 95.40±7.162 | t=5.094 |
| P=0.001 | P=0.001 | P=0.007 | |||||||
CFU: Colony forming unit, SD: Standard deviation, UV: Ultra violet
Table 3: Comparison of mean microbial CFUs before and after intervention (pre- and post-sanitization)
Comparison of the mean reduction in microbial contamination following intervention between different groups at different dilutions
The mean reduction in the total viable count following sanitization was significantly higher in the groups involving the use of UV rays and CHX compared with one achieved with saline in all the three dilutions [P < 0.003, Table 4]. However, the post-hoc comparison revealed no statistically significant difference between the CHX and saline at 10−6 serial dilution.
A statistically significant difference was observed in the mean reduction in total viable count between CHX and UV rays (P: 0.001) and between UV rays and saline (P: 0.001) at 10−6 and 10−7 serial dilution. However, no statistically significant difference was observed between CHX and saline at 10−6 and 10−7 serial dilutions.
Although a significant difference (P: 0.002) was observed between UV rays and the saline group, no such difference was seen among other groups at 10−8 serial dilution [Table 4].
| Groups | Mean difference (between pre- and post-sanitization) in microbial contamination at 10−6 serial dilution mean±SD |
Mean difference (between pre- and post-sanitization) in microbial contamination at 10−7 serial dilution mean±SD |
Mean difference (between pre- and post-sanitization) in microbial contamination at 10−8 serial dilution mean±SD |
|---|---|---|---|
| Chlorhexidine (A) | 111.60±35.487 | 133.40±34.432 | 150.60±33.400 |
| UV rays (B) | 254.00±2.986 | 239.40±18.007 | 205.00±30.408 |
| Saline (C) | 102.80±12.478 | 90.60±20.457 | 93.80±39.877 |
| Total | 156.13±73.596 | 154.42±68.633 | 149.80±57.835 |
| Statistical inference | F: 68.399 | F: 40.560 | F: 11.079 |
| df: 2 | df: 2 | df: 2 | |
| P: 0.001 | P: 0.001 | P: 0.003 | |
| Post-hoc results | A versus B: 0.001 | A versus B: 0.001 | A versus B: 0.129 |
| A versus C: 0.868 | A versus C: 0.067 | A versus C: 0.084 | |
| B versus C: 0.001 | B versus C: 0.001 | B versus C: 0.002 |
UV: Ultra violet, SD: Standard deviation
Table 4: Comparison of mean reduction in microbial contamination following intervention between different groups at different dilutions
Discussion
The toothbrush is a device designed to maintain the dental and overall oral health. The tooth brush plays a vital role in mechanical plaque control. It has been used in various forms and has proven to be efficient in removing plaque. Tooth brush, during oral use can get contaminated with microorganisms present in the mouth. It is a common practice to rinse the tooth brush with plain water after oral use and store it in a place near or in the bathrooms. Bacteria are more likely to grow in these moist and warm conditions. [11] Therefore, the storage and maintenance of the toothbrush after brushing has become increasingly important. In an attempt to decontaminate the toothbrushes, several chemical disinfecting solutions have been tried such as hexidine mouthwash, hydrogen peroxide, dettolin and cetylpyridinium chloride. Herbal products like 3% neem, 5% turmeric were also investigated for their effectiveness as antimicrobial solutions. Literature review suggests that chemical disinfectants and herbal products are effective in decontamination of tooth brushes. [12]
However, none of these studies have suggested an easy, economical and the most effective method for disinfecting a toothbrush. Hence, the present study is an attempt to compare the efficacy of 0.2% CHX gluconate solution, ultraviolet radiation and saline solution in the reduction of bacterial load from contaminated tooth brushes.
The tooth brushes in the present study were collected 7 days after oral use, and were subjected to microbial analysis at different dilutions.
The toothbrush collection time, i.e., 7 days in the present study can be compared to studies by Sogi et al. [13] and Bhat et al. [9] Different study intervals in the present study are based on the methodology employed in studies like Grewal and Swaranjit. [14]
Toothbrushes were randomly allocated to three intervention groups. In all the groups, the mean CFU at 10−6, 10−7 and 10−8 dilution were recorded and were in the range of 123–311 CFU’s. There was no statistically significant difference in the mean CFU’s before intervention (pre-sanitization) among the groups. All the toothbrushes were contaminated with bacteria after oral use. This finding suggests that the stored toothbrushes harbor different species of bacteria. Similar results have been described in the previous studies indicating that there exists a risk of colonization of bacteria on the toothbrushes after use. [15-17]
Microorganisms can not only colonize, but also retain on the toothbrushes for considerable time duration. Svanberg [7] in their study observed that heavily infected toothbrushes even after 24 h of use. Studies have shown that contaminated toothbrush can harbor streptococci that may cause pharyngitis or tonsillitis in children. [15] Furthermore, improperly cleaned or rinsed toothbrushes may lead to bacteremia. [12]
Toothbrushes can be a source or carrier of infection into the oral cavity. Therefore, it is useful to disinfect toothbrush at regular interval to prevent serious infections. The need for disinfection of used toothbrushes has been suggested by several authors using different methods like microwave irradiation, chemical and herbal agents. [9]
The present study used three disinfection or sanitization techniques like CHX solution, Ultraviolet irradiation and saline solution. Our results showed a significant reduction in mean bacterial count after the use of CHX solution, UV irradiation and normal saline solution at various dilutions. The difference was statistically significant before (pre-sanitization) and after intervention (post-sanitization).This suggests that the definite disinfection procedure is required for contaminated toothbrushes. Our findings with CHX solutions are in accordance with the results of Bhat et al. [18] and Balappanavar et al. [11] though both of the studies assessed only the reduction of S. Mutans count after disinfecting with CHX solution. CHX is the gold standard and exhibits broad spectrum antimicrobial effect. CHX gluconate has shown to inhibit bacterial count effectively in contaminated toothbrushes.
In the present study, 0.2% CHX gluconate solution was used, and toothbrushes were soaked for 12 h overnight. Though, studies using different concentration of CHX (Glass and Jensen) [5] and soaking times (Grewal and Swaranjit) [14] have also been conducted.
The findings in the UV radiation group reveal that it is more effective in the reduction of total viable count on toothbrushes compared to CHX solution and saline solution. UV light is capable of inactivating the microorganisms by disrupting the chemical bonds that hold the DNA atom. The toothbrushes were exposed to UV light for seven minutes as per manufacturer’s instructions. Studies have suggested that longer exposure to UV light can further lead to complete deactivation of microorganism. Study by Arrage et al. [19] suggests that some of the bacteria are tolerant to UV radiation. Several aerobic, Gram-positive, subsurface bacteria exhibited greater resistance to UV light. Although UV tolerance/resistance may also depend on physiological and behavioral traits, such as cell morphology, pigmentation and photo-toxicity. [19] Similar to our results Boylan et al. [20] have reported 86% reduction in total viable bacteria after irradiation with UV in toothbrush holder.
The present study demonstrated that saline solution, when used as disinfecting solution showed a significant reduction in the mean number of colony-forming bacterial units. This may be due to storage of toothbrushes in saline for 12 h. Storage conditions of toothbrushes are an important factor for bacterial survival (Dayoub et al.). [21]
In this study 0.2% CHX gluconate solution, UV rays and saline solution were all efficacious in reducing bacterial load on toothbrushes. However, UV toothbrush holder was found to be more efficacious, when compared with other groups. Hence, the present study concludes that UV light toothbrush holder is rapidly effective and nontoxic, and can be easily implemented. However, the UV light toothbrush holder is expensive and may not be that much cost effective. The cost factor also needs to be addressed before recommending the technique for a community at large. CHX solution is easily available and it is cost effective, when compared to UV light toothbrush holder. Therefore, chemical method of disinfection of toothbrushes using CHX may be recommended for toothbrushes, as a disinfecting solution in the developing countries.
The small sample size and lack of monitoring of the study participants is one of the limitations of the study. Another limitation is that the study did not consider specific microorganisms responsible in the causation of oral diseases. Nevertheless, it opens new avenues for further researches and contributes to the understanding of the existing literature.
Conclusion
Based on this study results, the following conclusions can be made.
• All the three methods are effective in toothbrush decontamination, but UV rays have greater efficacy followed by CHX and saline
• Overnight immersion of a toothbrush in CHX gluconate (0.2%) was also found to be highly effective in preventing microbial contamination
• Disinfection of tooth brush may prevent the occurrence of cross infection or re-infection of patients using a contaminated toothbrush
• UV rays toothbrush holder used in the study was expensive compared to other groups used in the study. Studies to find out the cost-effectiveness of UV chamber (toothbrush holder) may be recommended in the future.
Acknowledgments
First and foremost I offer my sincerest gratitude to my supervisor, Resp. Dr. Sudheer Hongal, who has supported me throughout my work with his patience and knowledge. The good advice and support of Dr. Vrinda Saxena and Dr. Manish Jain have been invaluable on both an academic and a personal level, for which I am extremely grateful. I am also thankful to Dr. Kuldeep Rana and Dr. Rahul Ganavadiya, who showed their kind concern and consideration regarding my research.
References
- Gujjari SK, Gujjari AK, Patel PV, Shubhashini PV. Comparative evaluation of ultraviolet and microwave sanitization techniques for toothbrush decontamination. J Int Soc Prev Community Dent 2011;1:20-6.
- Frazelle MR, Munro CL. Toothbrush contamination: A review of the literature. Nurs Res Pract 2012;2012:420630.
- Nelson-Filho P, Faria G, da Silva RA, Rossi MA, Ito IY. Evaluation of the contamination and disinfection methods of toothbrushes used by 24- to 48-month-old children. J Dent Child (Chic) 2006;73:152-8.
- Karibasappa GN, Nagesh L, Sujatha BK. Assessment of microbial contamination of toothbrush head: An in vitro study. Indian J Dent Res 2011;22:2-5.
- Glass RT, Jensen HG. More on the contaminated toothbrush: The viral story. Quintessence Int 1988;19:713-6.
- Cobb CM. Toothbrushes as a cause of repeated infections of the mouth. Boston Med Surg J 1920;183:263-4.
- Svanberg M. Contamination of toothpaste and toothbrush by Streptococcus mutans. Scand J Dent Res 1978;86:412-4.
- Turner LA, McCombs GB, Hynes WL, Tolle SL. A novel approach to controlling bacterial contamination on toothbrushes: Chlorhexidine coating. Int J Dent Hyg 2009;7:241-5.
- Bhat SS, Hegde KS, George RM. Microbial contamination of tooth brushes and their decontamination. J Indian Soc Pedod Prev Dent 2003;21:108-12.
- Sato S, Pedrazzi V, Guimarães Lara EH, Panzeri H, Ferreira de Albuquerque R Jr, Ito IY. Antimicrobial spray for toothbrush disinfection: An in vivo evaluation. Quintessence Int 2005;36:812-6.
- Balappanavar AY, Nagesh L, Ankola AV, Tangade PS, Kakodkar P, Varun S. Antimicrobial efficacy of various disinfecting solutions in reducing the contamination of the toothbrush-A comparative study. Oral Health Prev Dent 2009;7:137-45.
- Müller HP, Lange DE, Müller RF. Actinobacillus actinomycetemcomitans contamination of toothbrushes from patients harbouring the organism. J Clin Periodontol 1989;16:388-90.
- Sogi SH, Subbareddy VV, Kiran SN. Contamination of toothbrush at different time intervals and effectiveness of various disinfecting solutions in reducing the contamination of toothbrush. J Indian Soc Pedod Prev Dent 2002;20:81-5.
- Grewal N, Swaranjit K. A study of tooth brush contamination of different time intervals and comparative effectiveness of various disinfecting solutions in reducing toothbrush contamination. J Indian Soc Pedod Prev Dent 1996;14:10-3.
- Fischer H. Contaminated toothbrushes and pharyngitis. Arch Otolaryngol Head Neck Surg 1999;125:479.
- Sconyers JR, Crawford JJ, Moriarty JD. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc 1973;87:616-22.
- Schlein RA, Kudlick EM, Reindorf CA, Gregory J, Royal GC. Toothbrushing and transient bacteremia in patients undergoing orthodontic treatment. Am J Orthod Dentofacial Orthop 1991;99:466-72.
- Bhat PK, Badiyani BK, Sarkar S, Chengappa S, Bhaskar NN. Effectiveness of antimicrobial solutions on Streptococcus mutans in used toothbrushes. World J Dent 2012;3:6-10.
- Arrage AA, Phelps TJ, Benoit RE, White DC. Survival of subsurface microorganisms exposed to UV radiation and hydrogen peroxide. Appl Environ Microbiol 1993;59:3545-50.
- Boylan R, Li Y, Simeonova L, Sherwin G, Kreismann J, Craig RG, et al. Reduction in bacterial contamination of toothbrushes using the Violight ultraviolet light activated toothbrush sanitizer. Am J Dent 2008;21:313-7.
- Dayoub MB, Rusilko D, Gross A. Microbial contamination of toothbrushes. J Dent Res 1977;56:706.