Effects of ball-milling on PLGA polymer and its implication on lansoprazole-loaded nanoparticles
- *Corresponding Author:
Date of Received: 21-03-2011
Date of Modified: 15-04-2011
Date of Accepted: 17-04-2011
Available Online: 15-05-2011
Abstract
PLGA is a biodegradable polymer utilised widely in pharmaceutical research for the encapsulation of a wide range of drugs as nano particulate systems. This study investigates the impact of rotary ball milling on the physical properties of PLGA and its influence on nanoparticle formation prepared using the solvent displacement technique. By applying mechanical stress to the polymer and altering its physical appearance and molecular weight, the loading of lansoprazole within the nanoparticles was increased to 96%, with a reduction in particle size. The results indicate that rotary ball milling significantly reduces particle size, increases lansoprazole loading and improves the release profile for lansoprazole loaded PLGA nanoparticles.
Keywords
Milling, PLGA, polymeric nanoparticles, lansoprazole, characterisation
Introduction
The oral route for drug delivery remains the preferred route for drug administration for paediatrics and geriatrics, predominately due to its non-invasive nature. Additional advantages also include avoidance of discomfort associated with other forms of delivery, such as intravenous, its ease of convenience, good patient compliance, and low production cost [1-2].
In 2007 the European Medicines Agency (EMEA) [3] published a list of drugs recommended for studies into reformulation of off -patent paediatric medicinal products which included proton pump inhibitors. Lansoprazole is a proton pump inhibitor used to reduce the acidity of the stomach. It has a water solubility profile of 0.97 mg/L and a pKa of 8.5 (figure 1), and due to this high hydrophobic nature and formulation limitations it is currently only available as a solid dosage form. Following oral administration, lansoprazole acts in a number of ways, mainly by; significantly decreasing the basal acid output and increasing the mean gastric pH and the percent of time the gastric pH remains above 3 and 4; reducing meal-stimulated gastric output and secretion volume; reducing the pentagastrin-stimulated acid output; and inhibiting the secretion volume, acidity and acid output induced by insulin [4].
In order to improve the oral delivery of a hydrophobic drug in a liquid formulation, their association with a colloidal carrier, such as a polymeric nanoparticle is a preferred approach to increase bioavailability and prevent degradation in the acid environment of the stomach. Biodegradable nanoparticles are an emerging field in drug delivery. The polymers associated with this system enhance the therapeutic efficacy of the delivery system by site specific delivery with minimized side-effects. Nanoparticles are defined as having a particle size of <1000 nm. The drug is present in the dissolved, entrapped, encapsulated form or is attached to the polymer matrix [1,5]. Nanoparticles offer several advantages. They can deliver wide range of drugs hydrophilic as well as hydrophobic, can be administered via the oral route, target drug delivery to the specific parts of the gastro intestinal tract and can be prepared using a wide range of methods. Commonly studied polymers include polycaprolactone (PCL), poly (lactic-co-glycolic acid) (PLGA) and poly (lactic acids) (PLA). The type of polymer used can result in differences in the physiochemical characteristics of the nanoparticle formed such as particle charge and size, as well as modulating the drug release and biological behaviour [6-9].
In the current study, the biodegradable polymer utilised to form the matrix of the nanoparticle was PLGA (figure 2). PLGA is a polylactide, which has been deemed safe for human consumption [10]. This polymeric coating of nanoparticles not only prevents degradation but also aids in the regulation of drug release from the nanoparticles. It has been stated by Probha and Labhasetwar [11] that variation in the polymer-to-drug ratio, as well as the molecular weight and composition of the polymer that can modify the extent of release of the drug.
Many studies have been reported where the loading and sizing on nanoparticles have been recorded using pre-formed polymers, however limited research has highlighted the implications of modifying surface properties of the polymer prior to nanoparticle formation, and analysing its implications on its physical characteristics, drug loading and release profile. The advantage of dry milling the polymer prior to nanoparticle formation is that the surface characteristics of the polymer can be modified without any chemical modification. Planetary ball milling can be used to reduce particle size and in turn alter surface properties of materials by increasing surface area, porosity and shape.
The aim of this study was to ascertain the implications of milling of the polymer on the nanoparticle size, drug loading, physical properties and drug release, in comparison to a nanoparticle formulated using non milled polymer.
Materials and Method
Materials
Monobasic sodium phosphate, sodium hydroxide, sodium dodecylsulphate, sodium hydroxide pellets (NaOH), potassium bromide (KBr), dimethyl sulfoxide-d6 (DMSO-D6), Tetrahydrofuran, Lansoprazole, Pluronic F127, and Poly (lactic-co-glycolic acid) (PLGA) with a molecular weight of 8260 kDa and a lactide-glycolide ratio of 50:50 were all purchased from Sigma (UK).
Nanoparticle preparation using the solvent displacement technique
PLGA was dissolved in 10 mL of acetone to which 10 mg of lansoprazole was added and left to stir until lansoprazole had fully dissolved. The amount of polymer added was varied to produce the following polymer-to-drug ratios: 5:1, 6:1 and 7:1. The organic solution formed was added drop-wise into 10 ml of 0.25 % (w/v) Pluronic F127 solution in PBS, under magnetic stirring. The volume of nanosphere dispersion was then concentrated to 10 mL under reduced pressure. The final solution was then centrifuged at 3200 rpm for 30 minutes, to allow for the separation of the non-encapsulated lansoprazole from the encapsulated nanoparticles. The supernatant formed was separated for lansoprazole analysis via UV, and the pellets were re-dispersed in distilled water to produce a final concentration of 1 mg.mL-1 of lansoprazole.
Particle sizing and particle charge
Particle size of all of the formulations were analysed using a Zetasizer (Brookhaven). The sample was diluted by adding 50μL of sample in 2mL of distilled water in a cuvette. Values reported are the mean + SD of 3 different batches of each formulation. For particle size analysis the polydispersity index was also measured, this is a measure of the width of distribution calculated from the SD of the distribution divided by the mean value.
Zeta potential (surface charge) of all of the formulations were analysed using a Zetasizer (Brookhaven). The samples were diluted by adding 50μL of sample in 2mL of distilled water in a cuvette, to which a gold-plated zeta dip probe had been placed within. Values reported are the mean + SD of 3 different batches of each formulation
Planetary-ball milling
Using a planetary micromill (Pulveristte 7, Fritsch, Germany), 18 agate balls were placed into each of the planetary mill chambers, to which 3 g of the polymer is placed on top. The lid was secured in place ensuring the white rim was also in place to help seal the lid down, the chambers were then screwed securely in place. PLGA was milled for 1 hour at 200rpm.
Wettability profiling
Contact angle measurements were made using the Wilhelmy-plate method, using the QCT-100 programme. A glass coverslip was on both sides with the polymer and hung on a balance, and slowly lowered into a beaker containing distilled water at a rate of 0.2 mm/s. The advancing, receding and hysterisis contact angles of the particles was calculated using the QCT-100 software provided.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of the samples (lansoprazole, PLGA, milled PLGA, pluronic F127, and nanoparticle formulations) were obtained on an IR 200 spectrophotometer (UK). The samples were previously ground and mixed thoroughly with KBr and compressed.
Molecular weight analysis of milled polymers using gel permeation chromatography (GPC)
Using a PL-GPC 50 machine (A Varian, Inc. Company, by polymer laboratories), 3 mg of the milled polymer was dissolved in 1 mL of tetrahydrofuran, which was then injected into the GPC machine. Samples were analysed using the GPC/ SEC software.
Proton Nuclear Magnetic Resonance Spectroscopy (H1-NMR)
Lansoprazole-loaded milled and non-milled PLGA nanoparticles, physical mixture of milled and nonmilled PLGA and lansoprazole were prepared for NMR analysis. The spectra were acquired in DMSO- D6 solution at room temperature. All NMR measurements were done with standard Bruker pulse sequences.
Differentail Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) (Perkin- Elmer, Wellesley, USA) was used to study the glass transition temperatures (Tg) and melting points (Tm). Lansoprazole, pure non milled PLGA and milled PLGA were prepared for DSC analysis. The samples were heated from 0 to 240°C at a rate of 10°C/min, with a nitrogen purge of 20 ml/min.
In Vitro Release Studies in Simulated Fasted State
The release of lansoprazole from the nanoparticles was studied in the fasted state in accordance with the United States Pharmacopoeia (USP) 31- standard. All dissolution vessels were wrapped in foil to prevent photodegradation of lansoprazole as it was being released from the nanoparticles into the medium. In Vitro release was carried out in dissolution baths at 37°C. The buffer consisted of an acid stage containing 500 mL 0.1 M HCL at pH 0.3. The nanoparticles were placed in rotating baskets at 75 rpm for 1 hour in the acid solution. After which samples were placed into a 900 mL phosphate buffer at pH 6.8. The buffer consisted of monobasic sodium phosphate, sodium hydroxide and sodium dodecylsulphate (16.35 g, 7.05 g and 3 g respectively in 1 L of distilled water). The buffer is adjusted to pH 6.8 using 10 M NaOH. At set time points (5, 10, 15, 30, 45, 60, 90, 120, 180, 240, 360, 1440 and 2880 minutes 0-48 hours) 5 mL sample of the release medium was withdrawn, following this 5 mL of fresh phosphate buffer was added to the dissolution baths in order to maintain sink conditions. The 5 mL samples of release medium were then assayed at 285 nm for drug recovery using the UV spectrophoto meter. The drug release experiments were carried out in triplicate.
Results and Discussion
The influence of PLGA concentration on nanoparticle characteristics
PLGA-to-lansoprazole concentrations of 5:1, 6:1, and 7:1 (w/w) in the organic phase were used to evaluate the effect of varying the polymer concentration on crucial characteristics of the prepared nanoparticles. The mean particle size was found to increase significantly by increasing PLGA concentration with no significant change in the polydispersity index (table 1). The results are in line with previously reported literature which found that lower polymer concentration resulted in relative lowering of viscosity of the inner organic phase which prevents formation of polymer chain aggregates resulting in reduced coalescence and ultimately smaller particle sizes [12]. On the other hand an increase in polymer concentration results in higher viscosity, which synergistically results in a larger mean particle size. This could be potentially attributed to the relatively lower flexibility of polymer chains to condense into smaller nanoparticles (due to increase in viscosity of the organic phase) during solvent evaporation and the presence of greater number of polymer chains within the system.
| Polymer-to-lansoprazoleratio | Particle size (nm) | PI | Zeta Potential (mV) | PH | LSP retention (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLGA | MPLGA | PLGA | MPLGA | PLGA | MPLGA | PLGA | MPLGA | PLGA | MPLGA | |
| 5:1 | 259.8 ± 7.41 | 237.7 ± 7.44 | 0.13 ± 0.05 | 0.07 ± 0.06 | -25.89 ± 3.22 | -14.78 ± 4.53 | 6.10 ± 0.25 | 7.21 ± 0.06 | 80.55 ± 0.64 | 92.37 ± 1.31 |
| 6:1 | 272.0 ± 1.56 | 257.6 ± 8.40 | 0.12 ± 0.04 | 0.10 ± 0.01 | -37.31 ± 6.44 | -20.56 ± 4.27 | 6.76 ± 0.12 | 6.85 ± 0.10 | 80.01 ± 0.65 | 96.30 ± 0.93 |
| 7:1 | 292.5 ± 21 | 267.6 ± 1.05 | 0.06 ± 0.03 | 0.14 ± 0.09 | -37.64 ± 4.65 | -30.60 ± 2.63 | 7.11 ± 0.05 | 7.16 ± 0.05 | 80.49 ± 0.54 | 95.38 ± 3.23 |
Table 1: Characterisation of non-milled (PLGA) and milled PLGA (mPLGA) with increasing polymer concentration
Measurement of surface charge of the nano particles showed the presence of an anionic surface. The negative zeta potential of the nanoparticles (Table 1) is possibly due to the presence of terminal carboxylic groups on the polymer chains [13-14] which ensures a high energy barrier preventing particle aggregation and improving product stability [15]. In another study it was found that a zeta potential of –25 mV allows greater stabilisation of nanoparticles due to the higher repulsive forces preventing aggregation upon ageing, as compared with a colloidal system having a zeta potential of –10 mV [16].
Despite the differences in mean particle size, an increase in PLGA concentration within the formulation did not have any effect on drug loading (Table 1). The drug entrapment efficiency remained unaltered at 80 % upon changes in the concentration of the polymer. The next stage of the investigation focussed on investigating alternative approaches to promote drug loading. The primary criteria included methods/approaches that would require no heat production or pH variations after lansoparazole addition as it is sensitive to degradation by heat and low pH. To this end alteration of excipient properties was the most suitable approach and dry ball milling of the polymer was carried out to study its influence on formulation properties and drug loading.
The influence of milling on nanoparticle characteristics
The advantage of dry milling PLGA prior to nanoparticle formation was that the physiochemical characteristics, such as molecular weight, surface characteristics of the polymer can be modified without applying any stresses to the process of nanoparticle preparation. For example using a shearing force (such as high-pressure homogenisation) to reduce the nanoparticle size and to promote drug loading can generate heat that may affect drug stability [17].
Characterisation results of nanoparticles loaded with lansoprazole that were produced from milled PLGA are summarised in Table 1. The results clearly showed significant decreases in the nanoparticle size and in zeta potential (more negative charge) at all concentrations of the milled PLGA compared to similar concentrations of nanoparticles based on the non milled polymer (Table 1). Similarly determination of drug loading also resulted in significant improvement in drug entrapment upon incorporation of milled polymer when compared to non milled polymer. Inclusion of 6:1 ratio of milled polymer against the non milled polymer resulted in a 16 % increase in drug loading from an initial 80 % to 96%. This may be attributed to the reduced size of the nanoparticles, which allow for more efficient encapsulation by providing a larger surface area for the drug to interact with, in comparison to the larger nanoparticles. The increase in zeta potential (less negative charge) can be a direct result of the reduction in particle size (as less polymer chains form single nanoparticle) and/or as a consequence to the increase in lansoprazole loading, which has a positive charge, and consequently may adsorb on the surface of the nanoparticles and reduce its negative charge.
Mechanical milling of polymers can induce structural changes that may cause degradation [18], structural modifications, in addition to molecular weight [19] and alterations in thermal properties [20]. To the best of our knowledge the effects of mechanical milling on the physiochemical properties of PLGA have not been studied before. To investigate the possible modifications on the nature of PLGA and correlate this to the increase in encapsulation efficiency reported above, various chemical and physical analyses were performed and compared to the non-milled polymer.
Influence of milling on the physicochemical properties of PLGA
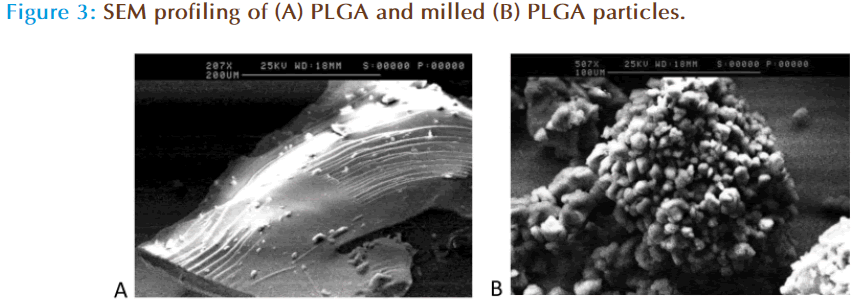
Scanning electron microscopy (SEM) profiling of PLGA polymer before and after milling is shown in Figure 3. Prior to ball-milling PLGA has a large smooth glassy appearance, whereas ball milling of the polymer showed changes in the appearance of PLGA resulting in the formation of smaller particles aggregated together to form a bigger globular structure. Research in the area of material science has shown that milling results in the formation of amorphous phase and nano porous structures within the surface which results in increase in surface area [21, 22]. The formation of nanoporous surface was attributed to the high energy impact that occurs when the surface of the material comes into direct contact with the rotating balls which results in the formation of nano scale pores due to the heat generated between the two surfaces. To further investigate the differences in the data obtained from SEM studies, wettability profiling of the milled polymer was compared against the non milled polymer.
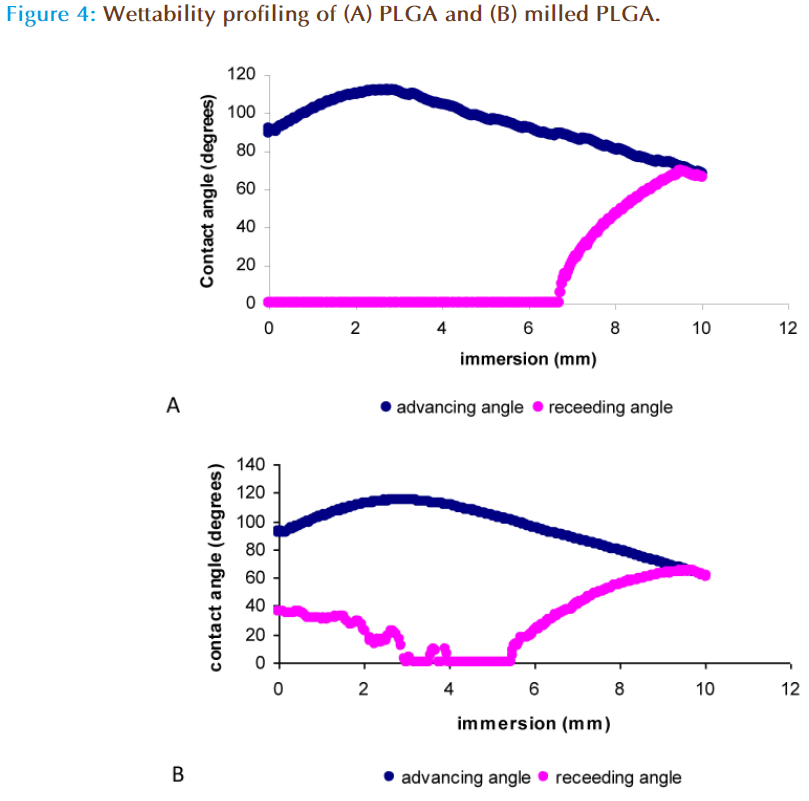
The wettability of a substance is determined by measurement of its advancing and receding angle and the difference between the two angles is used to calculate the angle of hysterisis. The contact angle is a quantitative measure of the wetting of a solid by a liquid, with values less than 90° indicating that the liquid wets the solid completely. An angle greater than 90° indicates that the solid material has poor wettability and is hydrophobic in nature [23-24]. PLGA is a hydrophobic polymer, as indicated by Figure 4a in which after complete immersion of the polymer in distilled water, the advancing contact angle was 67.17 ± 0.43° and the receding angle was 90.00 + 0.00° indicating that the polymer was not ‘wetted’ and is thus hydrophobic. The milled polymer displayed an advancing angle of 51.71 ± 10.97° with incomplete wetting during the receding angle (no angle was measurable), indicating the polymer hydrophobicity was altered [25] (Figure 4b). This modification in the wetting properties can be attributed to the smaller particles size of the milled polymer, which may offer larger surface to interact with water or due to modifications in the chemical structure of PLGA after the impact of milling. This was further investigated by molecular weight analysis, Fourier Transform Infra-Red spectroscopy (FTIR), H1-NMR and DSC.
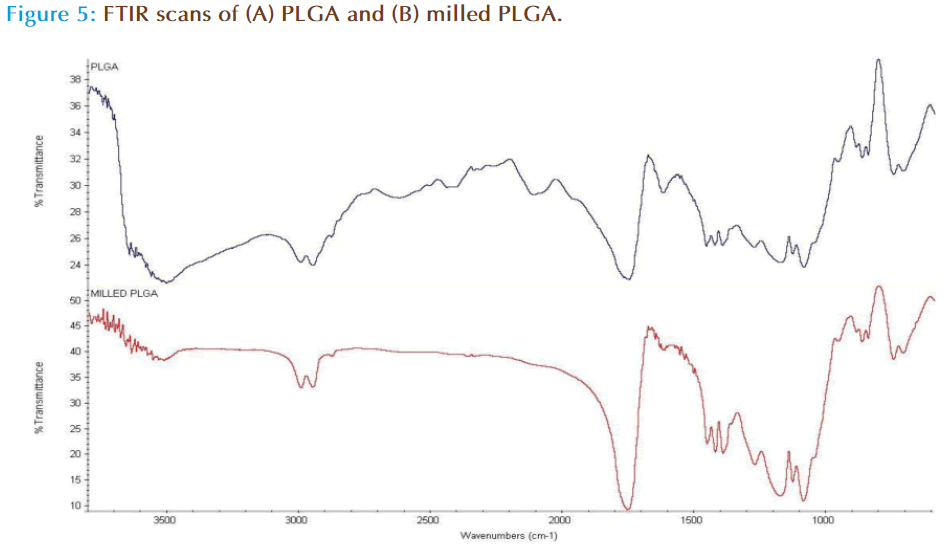
FTIR was carried out in order to assess bond integrity from milling the polymer. The structural composition of PLGA is displayed in Figure 2, which shows that the weakest of the bonds is the O-H bond. Milling of the polymer possibly results in bond breaking at O-H which results in retention of 50:50 ratio of lactic and glycolic monomers but in turn altering the total polymer chain length as evidenced by molecular weight measurements (Table 2). The increase in molecular weight is possibly due to scissoring, mass transfer and cross linking events in the polymer chain [26] in response to the mechanical action of milling on the polymer surface.
| Polymer | Mn | Mw | PDI (Mw/Mn) |
|---|---|---|---|
| PLGA | 5548 | 8260 | 1.4888 |
| Milled PLGA | 6301 | 8356 | 1.3261 |
Table 2: Molecular weights of PLGA using gel permeation chromatography (GPC).
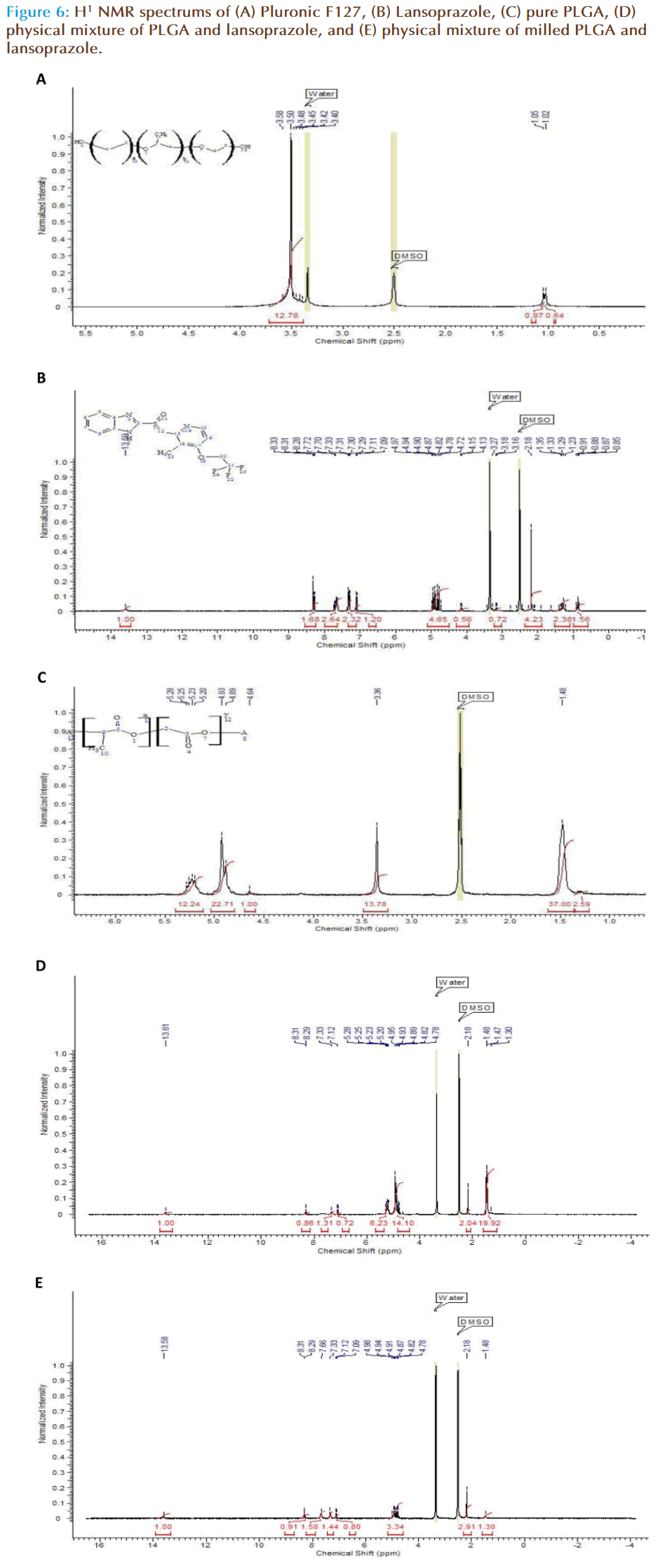
H1-NMR was carried out in order to establish a structural change within the polymer chain according to the number of protons present. To some extent the scope and limitation of analysis of polymer composites using 1H-NMR spectroscopy depends upon the appearance of the non-superimposed signals of the components in the spectra. Therefore the first stage was to highlight the spectra of the pure components. With F127 the following data was obtained (Figure 6a), a sharp singlet peak is obtained at 3.50 ppm, which has a broad base. The integration calculation indicates 12 H-ions to be present within this peak, thus the singlet corresponds to the main body of the F127 chain including the methyl side chain. At 1 ppm a doublet peak is observed which corresponds to the OH- groups present at the end of the chains. The NMR spectrum for lansoprazole shows; a single peak at 13.58 ppm corresponding to the N-H chain on the cyclic amide group, a set of 6 peaks at 4.82 ppm corresponding to the cyclic chain of lansoprazole and a triplet peak at 7.70ppm corresponding to the CH3- methyl side group on the amide cyclic chain (Figure 6b). The NMR spectrum for PLGA shows a large peak at 1.48 ppm corresponding to 37 H-ions, a large single peak at 3.36 ppm corresponding to approximately 14 H-ions and a doublet peak at 4.93 ppm corresponding to 23 H-ions and a further broad peak at 5.23 ppm indicating 12 H-ions are present, in total 1 polymer chain potentially holds 86 H-ions.
By eliminating the known spectral peaks of the individual components of the nanoparticle, any interaction occurring can be determined. The NMR spectrums for the physical mixture of non-milled and milled PLGA with lansoprazole were distinctly different. The non-milled PLGA displayed a small single peak at 13.58 ppm that represents the -N-H group of the lansoprazole structure. Also at 8.29 ppm a small doublet peak was observed with further two smaller single peaks at 7.33 and 7.12 ppm. Multiple peaks were seen at 4.93 and 5.20 ppm equalling to 14 and 6 H-ions respectively. When the physical mixture of milled PLGA and lansoprazole was analysed, it was observed that although the peak specific to –N-H is present at 13.58 ppm, several other peaks are either not present or have a lower number of H-ions designated to that particular peak. One particular example is the 20 H-ion large peak at 1.47 ppm, which is now representative of 1 H-ion. The NMR scans demonstrate significant differences between the physical mixture of milled and non-milled PLGA with lansoprazole.
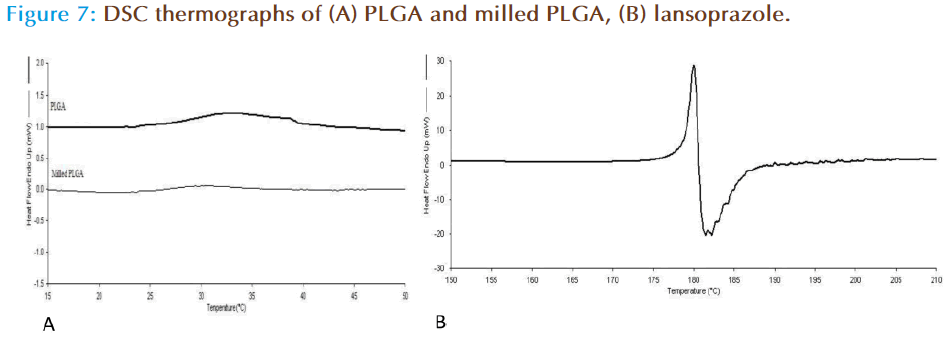
DSC was carried out in order to establish the thermodynamics of the milled and non milled PLGA polymers. Pure PLGA displayed a transition (Tg) at 33°C, with the milled polymer having a reduced Tg at 29°C (Figure 7a). This is possibly indicative of the milling process weakening the polymer chain bonds through repetitive impaction, and ultimately resulting in less energy. Investigation of lansoprazole (drug alone) thermal analysis showed that the drug exhibits a Tm at 179°C with an immediate degradation event at 180°C (Figure 7b). On the other hand the thermal behaviour of lansoprazole loaded nanoparticles prepared from milled as well as non milled powders, there was no melting peak detected for the drug suggesting that the drug was converted from crystalline into amorphous form during the formulation of nanoparticles (as the drug was dissolved in organic phase in which it was completely soluble).
The physicochemical analysis carried out in this study suggested that most dramatic effect of milling on PLGA was observed on the physical aspects of the polymer including its molecular weight and surface characteristics. It has been previously described that the energy transferred between the agate balls and powder during the milling process, produced a reduction in particle size and a modification of its physical appearance such as formation of stress marks, new surface composition and possibly a mass transfer [27-28]. Thus, the enhanced encapsulation efficiency of PLGA after milling can be attributed to one or more of the changes that have been described above and particularly increase in average molecular weight, reduction in particle size and/or alteration of wetting properties.
In vitro release studies
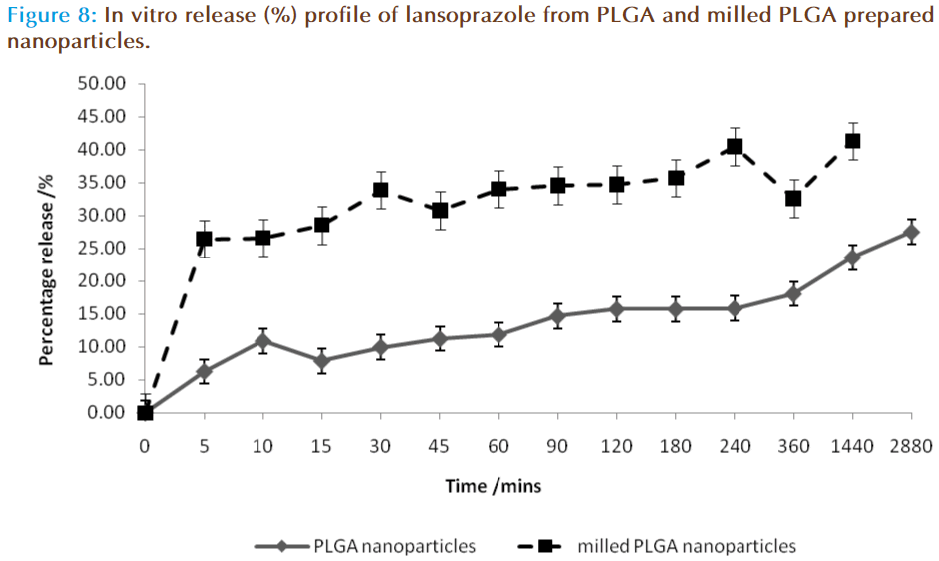
The in vitro release of lansoprazole from the nanosphere dispersions is displayed in figure 8. The release study was carried out in two stages, the first was in 0.1M HCL aimed at mimicking the acid in the stomach in the fasted state, and the second phase was in buffer at pH 6.8, aimed at mimicking the conditions within the small intestine. In the acid phase 25-35% of lansoprazole was released from the nanosphere preparation, with milled PLGA nanospheres resulting in a higher release profile of 34-44 % release. In the buffer stage, this trend continued in which the release rate of lansoprazole from milled PLGA nanoparticles was significantly higher than the release obtained from standard PLGA nanoparticles, with milled PLGA displaying a higher burst effect within the first 5 minutes (26.49 ± 17.53 %) as compared with the PLGA nanoparticles (6.28 ± 5.64 %). This burst affect is attributed to either weakly bound or adsorbed lansoprazole on the surface or within the outer layers of the nanoparticle [29-30]. After this initial burst release of lansoprazole, the drug was being released from the nanospheres at a steady increase. At the 48-hour mark, in the buffer phase 27.56 ± 14.05 % and 41.34 ± 19.82 % of lansoprazole from PLGA and milled PLGA nanoparticles had been released respectively. It was concluded that approximately 33.84 ± 19.69 % of lansoprazole was released in total from the PLGA matrix system, and 67.83 ± 37.35 % from milled PLGA system.
Nanospheres release drugs in one of two ways: diffusion or matrix erosion, the method of release depends on the rate of matrix erosion occurring. In the present study lansoprazole was found to be diffusing through the matrix at a faster rate than the polymer was degrading, and thus the rate of release in this system was concluded to be controlled by a diffusion process.
The rate of release was also controlled by the particle size of the system. The milled PLGA produced smaller nanoparticles, thereby increasing their surface area-to-volume ratio. Thus the majority of lansoprazole are located nearer to the polymeric particle surface and hence leading to a faster drug release. The non-milled PLGA nanoparticles displayed a larger particle size, and thus have a larger matrix system allowing more lansoprazole to be encapsulated within the one particle. In a larger polymeric particle, longer time is required to degrade the polymer in order to release all of the lansoprazole particles, thus resulting in a slower release profile [30-31]. The rate of release of lansoprazole can therefore be concluded to be directly related to particle size and the rate of drug diffusion.
Conclusion
In this study, solvent displacement technique was used for the production of spherical lansoprazoleloaded matrixes, composed from the PLGA biodegradable polymer. The influence of mechanical milling of polymers prior to nanoparticle preparation resulted in differences in drug encapsulation and release.
The process of milling not only changed the molecular weight of the polymer but also its physical aspects, making it less glassy in structure and more globular. This in turn resulted in reduced particle size and increased drug loading.
Preparative polymer variables such as molecular weight and concentration are important factors for the formation of PLGA nanoparticles. The release kinetics of lansoprazole were governed by the initial drug loading, higher the initial drug loading resulted in a faster drug release profile. The process of milling resulted in milled PLGA having a high drug loading capacity and thus the release of lansoprazole was faster than from the non-milled polymer.
References
- Rieux AD, Fievez V, Garinot M, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release. 2006; 116: 1-27
- Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int. J. Pharm. 2006; 317: 61-68
- European Medicines Agency. Updated priority list — revised for studies into off-patent paediatric medicinal products. 2007. Available at www.emea.europa.eu
- Barradell LB, Faulds D, McTavish D. Lansoprazole. A review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficiency in acid-related disorders. Drugs. 1992; 44(2): 225-250.
- Galindo-Rodriguez SA, Allemann E, Fessi H, et al. Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit. Rev. Ther. Drug Carr. Syst. 2005; 22: 419–464.
- Quintanar-Guerrero D, Allémann E, Fessi H, et al. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev. Ind. Pharm. 1998; 24: 1113-1128.
- Barichello JM, Morishita M, Takayama K, et al. Encapsulation of hydrophilic and lipophillic drugs in PLGA nanoparticles by the nanoprecipitation method. Drg. Dev. Ind. Pharm. 1999; 25(4): 471-476.
- Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid St. M. 2002; 6: 319-327.
- Teixeira M, Alonso MJ, Pinto MMM, et al. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur. J. Pharm. Biopharm. 2005; 59: 491-500.
- Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorgan. Med. Chem. 2009; 17: 2950-2962.
- Prabha S, Labhasetwar V. Critical Determinants in PLGA/PLA Nanoparticle- Mediated Gene Expression. Pharm. Res. 2004; 21: 354-364.
- Khandai M, Chakraborty S, Sharma A, et al. Preparation and evaluation of algino-sericin mucoadhesive microspheres: An approach for sustained drug Delivery. J. Adv. Pharm. Res. 2010; 1: 48-60.
- Verger MLL, Fluckiger L, Kim LI, et al. Preperation and characterization of nanoparticles containing antihypertensive agent. Eur. J. Pharm. Biopharm. 1998; 46: 137-143.
- Govender T, Stolnik S, Garnett MC, et al. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control. Release. 1999; 57: 171-185.
- Benita S, Levy MY. Submicron emulsions as colloidal drug carriers for intravenous administration:comprehensive physiochemical characterization. J. Pharm. Sci. 1993; 82: 1069-1079.
- Muller RH. Charge determinations. In: Muller RH, ed. Colloidal carriers for controlled drug delivery and targeting, modification, characterization and in vivo. Florida, USA: CRC Press; 1991: 57-97.
- Date AA, Patravale VB. Current strategies for engineering drug nanoparticles. Curr. Opin. Colloid. In. 2004; 9: 222-235.
- Smith AP, Shay JS, Spontak RJ, et al. High-energy mechanical milling of poly(methyl methacrylate),polyisoprene and poly(ethylene- altpropylene). Polymer. 2000; 41: 6271–6283.
- Gunther-Schade K, Castricum HL, Ziegler HJ, et al. Free Volume Changes in Mechanically Milled PS and PC Studied by Positron Annihilation Lifetime Spectroscopy (PALS). Polym. Eng. Sci. 2004; 44(7): 1351-1359.
- Castricum HL, Yang H, Bakker H, et al. A Study of Milling of Pure Polymers and A Structural Transformation of Polyethylene. Mater. Sci. Forum. 1997; 235: 211-216.
- Chen Y, Gerald JF, Chadderton LT, et al. Nanoporous carbon produced by ball milling. Appl. Phys. Lett. 1999; 74(19): 2782-2784.
- Oberlain A. TEM studies of carbonization and graphitization. In: Thrower PA, Ed. Chemistry and physics of carbon. New York, USA: Marcel Dekker; 1989: Vol. 22, pp 94.
- Yoon JY, Garrell RL. Biomolecular adsorption in microfluidics and nanofluidics. In: Li D, Ed. Encylopedia of microfluidics and nanofluidics. New York, USA: Springer Reference; 2006: Vol. 1. pp 69.
- Park JM, Wang ZJ, Kwon DJ, et al. Interfacial evaluation and hydrophobicity of multi-funcational Ni-nanopowder/epoxy composites for self-sensing and actuation. Smart. Mater. Struct. 2010; 19: 1-8.
- Hermitte L, Thomas F, Bougaran R, et al. Contribution of the comonomers to the bulk and surface properties of methacrylate copolymers. J. Colloid. Interf. Sci. 2004; 272: 82-89.
- Smith AP, Spontak RJ, Ade H. On the similarity of macromolecular responses to high-energy processes: mechanical milling vs. Irradiation. Polym. Degrad. Stabil. 2001; 72: 519–524.
- Suryanaryana, C.. Mechanical alloying and milling. Prog. Mater. Sci. 2001; 46: 1-184.
- Pilloni M, Nicolas J, Marsaud V, et al. PEGylation and preliminary biocompatibility evaluation of magnetite-silica nanocomposites obtained by high energy ball milling. Int. J. Pharm. 2010; 401: 103-112.
- Magenheim B, Levy MY, Benita S. A new in vitro technique for the evaluation of drug release profiles from colloidal carriers – ultrafiltration technique at low pressure. Int. J. Pharm. 1993; 94: 115-123.
- Singh R, Lillard Jr. JW. Nanoparticle-based targeted delivery. Exp. Mol. Pathol. 2009; 86: 215-223.
- Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-coglycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 9028: in vitro characterisation and in vivo evaluation. J. Control Release. 2001; 70: 353-363.