Effect of taxol from Pestalotiopsis mangiferae on A549 cells-In vitro study
- *Corresponding Author:
- Govindarajan Kathiravan
Department of Biotechnology, VELS University, Pallavaram, Chennai-600 117
E-mail: gkathir72@gmail.com
Date of Received: 03-01-2010
Date of Modified: 22-01-2010
Date of Accepted: 02-02-2010
Available Online: 15-02-2010
Abstract
Pestalotiopsis mangiferae Coelomycete fungi were used to examine the production of taxol. The taxol isolated from this fungus is biologically active against cancer cell lines were investigated for its antiproliferative activity in human Non Small Cell Lung Cancer A549 cells. The results showed that the methylene chloride extrac- tion of Pestalotiopsis mangiferae inhibited the proliferation of A 549 cells as measured by MTT and Trypan blue assay. Flow cytometric analysis showed that methylene chlo- ride extraction of Pestalotiopsis mangiferae blocked cell cycle progression in G0/G1 KEyWOrDS: Pestalotiopsis mangiferae; taxol; MTT; Trypan blue; G0/G1; LDH; apoptosis phase. In addition fungal taxol induced A549 cell apoptosis as determined by propid- ium iodide staining. Further the percentage of LDH release was increased at increasing concentrations which is a measure of cell death. The levels of sialic acid levels and DNA, rNA and protein levels were decreased after treatment with methylene chloride extraction of Pestalotiopsis mangiferae.We suggests that methylene chloride extrac- tion of Pestalotiopsis mangiferae might be considered for future therapeutic applica- tion with further studies against lung cancer.
Keywords
Pestalotiopsis mangiferae; taxol; MTT; Trypan blue; G0/G1; LDH; apoptosis
Introduction
Lung cancer is the leading cause of cancer death in the world and Non Small Cell Lung Carcinoma (NSCLC) accounts for approximately 75–85% of these cancers. Non small cell lung cancers commonly develop resistance to radiation and chemotherapy, and they often present at stages beyond surgical remedy. Since current treatment modalities are inadequate, novel therapies are necessary to reduce the effects of the increasing incidence in pulmonary neoplasm (Chen et al., 1995; Park et al., 2001).
Taxol, a diterpene was originally isolated from the bark of Pacific Yew tree (Taxus brevifolia) more then two decades ago and has proved to possess an anticancer activity. The US National Cancer Institute, in collaboration with Bristol Myers Squibb Co and other workers have demonstrated the efficacy of taxol against certain human cancer. Its mode of action is unique in that it inhibits mitosis through enhancement of polymerization of tublin and consequent stabilization of microtubles during the process of cell division. However, a complete treatment for the patient requires 2 grams of taxol, administered several times and many months. To obtain 1kg of taxol requires about 10,000 kg of bark, and several thousand tresses must be cut to procure this quantity of bark. This scarcity of taxol and the ecological impact of harvesting it encouraged scientists to find alternative methods using microorganisms. A hypomycetous fungus namely Taxomyces andreanae on Taxus sp., could produce taxol. A coelomycetous fungus, pestalotiopsis microspora, an entophyte from inner bark of Taxus wallachiana produced taxol in culture. Keeping in this mind, an attempt has been made to examine the production of taxol by some other coelomycetous fungus as well. The taxol isolated from these fungi is biologically active against cancer cell lines. In order to the price of taxol and make it more available, a fermentation process involving a microorganisms would be the most desirable means supply. It was first discovered by Strobel et al (1997)., that the fungus Taxomyces andreanae could produce taxol, through the yield was low. (Strobel et al., 1996) showed that Pestalotiopsis microspore isolated from the bark of Taxus wallachiana produced taxol in mycelial culture. This work prompted us to continue the search for the taxol production from fungal sources.
Apoptosis has been characterized as a fundamental cellular activity to maintain the physiological balance of the organism. It is also involved in immune defense machinery (Hengartner, 2000) and plays a necessary role as a protective mechanism against carcinogenesis by eliminating damaged cells or abnormal excess cells proliferated owing to various chemical agents induction (Brown and Wooters, 1999). Emerging evidence has demonstrated that the anti cancer activities of certain chemotherapeutic agents are involved in induction of apoptosis, which is regarded as the preferred way to manage cancer.
Materials and Methods
Source of chemicals
Fetal bovine serum (FBS), Penicillin G, Streptomycin and amphotericin B were obtained from Hi Media. Dimethylsulfoxide (DMSO) and RPMI–1640 were purchased form King Institute of Preventive Medicine. MTT and propidium iodide was purchased from SRL, Laboroties India. All other chemicals used were of analytical grade.
Fungal material and extract preparation
The general laboratory techniques followed in the course of the present investigation were as outlined by Booth (1971). The test fungi used in the present study were grown in Erlenmeyer flasks containing 500 ml MID medium supplemented with 1 gram of soytone L-1 (Pinkerton and Strobel, 1976) for taxol production. Three mycelial agar plugs (0.5 cm) were used as inoculums. The organisms were grown at 24±2°C statistically for 3-4 weeks.
Culture media (Strobel, 1996)
MID medium was supplemented with soytone, sucrose -30.0 g/l, (CHOH.COONH4) 2-5.0 g, Yeast extract -0.5g, Soytone-1.0g, Ca2(NO)3-280 mg, KNO3-80 mg, KCl-60 mg, MgSO4-360 mg, NaH2PO4-20 mg, H3BO3-1.4 mg, MnSO4-5.0 mg, ZnSO4-2.5 mg and KI-0.7 mg.
Extraction of taxol
Extraction of taxol was performed according to Strobel et al. (1996). After incubating the fungal culture for 3-4 weeks, the culture filtrate was passed through four layered cheesecloth. In order to avoid fatty acid contamination of taxol, 0.25g of NaCO3 was added to the filtrate. The culture fluid was extracted with two equal volumes of methylene chloride and the organic phase was evaporated to dryness under reduced pressure at 35°C.
2.2. Cell culture
A549 was obtained from NCCS Pune, India. A 549 cells were cultured in Ham’s F12k medium containing 10% new born calf serum containing 100μl/ml of penicillin and streptomycin 100μl/ml. Cells were maintained in a humidified atmosphere of 5% Co2 incubator at 37°C, until confluency stage is attained. The medium is replaced every two days and maintenance is in strictly accordance with the standard methods. The cells were dissociated with TPVG in Phosphate buffered solution.
Light microscopy
After 48 hours incubation with methylene chloride extract of fungal taxol, the A-549 cells were washed with PBS.The cells were observed for morphological changes under light microscopy at 100X and photographed.
Cell viability assay
Inhibition of cell proliferation by methylene chloride extract of fungal taxol, was measured by MTT assay as described (Mossmann, 1983). Briefly, cells were plated in 24 well culture plates (1x106 cells/ well). After 24 hrs incubation, cells were treated with crude extract (10μgs, 25 and 50 μgs) for 48 hrs. Fifty microlitres of MTT was added and the reading was taken at 570nm after lysing in isopropanol.
Trypan blue exclusion studies
The viability of cells were assessed by trypan blue exclusion studies for various concentrations (10, 25 and 50μgs/ml) at different time intervals 12, 24 and 48 hrs by the standard method (Moldeus, 1978).
Assay of lactate dehydrogenase (EC 1.1.1.27)
The activity of lactate dehydrogenase was assayed by the method described (King, 1965 a).
Assay of sialic acid levels
Sialic acid level were determined as according to the method described(Warren, 1959).
Estimation of macromolecules
Protein was estimated by the method of Lowry et al (1951). DNA was estimated as according to the method of Burton (1956). RNA was estimated by the method of Rawal et al (1977).
Measurement of apoptosis by propidium iodide staining
The induction of apoptosis by methylene chloride extract of fungal taxol was assayed by the propidium iodide staining. For determination of apoptosis by propidium iodide staining, the A549 cells were treated with methylene chloride extract of fungal taxol and stained with propidium iodide (50 μgs/ml). This is then viewed under the fluorescent microscope.
Flow cytometry
This analysis was carried according to the method described by Nicotelli et al (1991). 10 lakh cells of A549 were cultured in 6 well plates and incubated for 48 hours to obtain a monolayer. To this culture, the drug was added at 50μgs concentrations and incubated for a period of 24 hours. The cellular morphology was viewed once every few hours. After the incubation period, the media was removed and the cells gently washed with PBS. The cells were then trypsinised. The suspension was then centrifuged at 3000 rpm for 5 minutes and supernatant was dis carded. The pellet obtained was gently washed with ice cold PBS and resuspended. To this 40μl of 2mg/ ml propidium iodide was added and kept for 4 hours of incubation at 4oC. This was subjected to flow cytometric analysis.
Results
TLC analysis was carried out on Merck 1mm(20x20 cm) silica gel plate developed in solvent A (chloroform:methanol,7:1v/v) followed by solvent B (Chloroform: Acetonotrile, 7:3v/v), solvent C (Ethyl acetate: 2-propanol, 95:5,v/v) solvent D (Methylene chloride: Tetrahydrofuran,6:2v/v) and solvent E (Methylene chloride: Methanol:Dimethylf ormamide,90:9:1v/v/v) respectively. The area of the plate containing putative taxol was carefully removed by scrapping off the silica at the appropriate Rf and eluted with acetonitrile. Taxol was detected with 1% w/v vanillin/sulphuric acid reagent after gentle heating (Cardellina, 1991). It appeared as a bluish spot that faded to dark grey after 24 h.
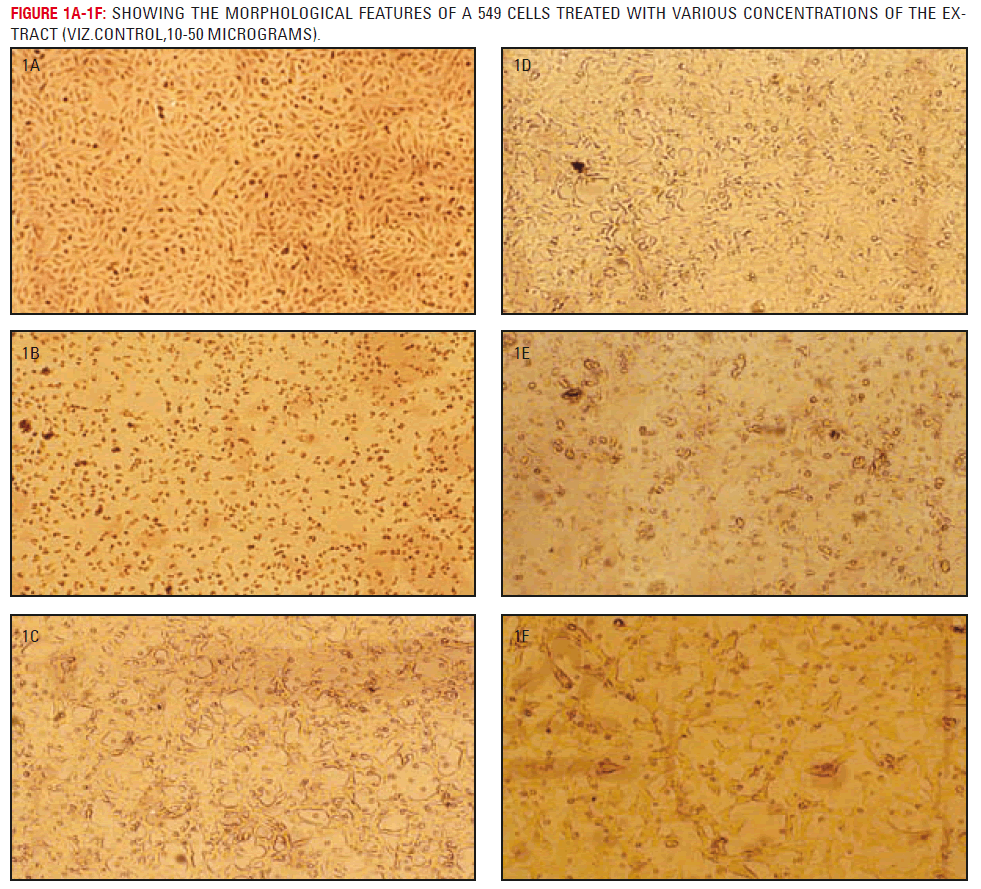
Architecture of untreated A549 cells show a typical spindle shaped cells observed under a light microscope. It exhibited typical carcinoma type morphology with a uniform monolayer (figure 2.1). In vitro incubation of A-549 cells with the methylene chloride extract of fungal taxol (1-5μgs) showed remarkable morphological alterations (figure 2.2-2.6) and cell death at the end of 48 hours characterized by disruption of monolayer. It caused a dose dependent cytolytic and nuclear change in cell morphology. Cell mitosis were scarce compared to untreated A549 cells.
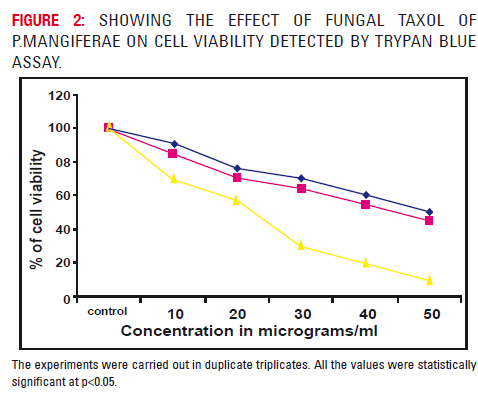
Figure 3 showed the effect of methylene chloride extract of fungal taxol on A549 cell viability (Trypan blue uptake) after 12, 24, 48 hrs incubation. It was inferred that the incubation of methylene chloride extract of fungal taxol had led to marked decrease in viable cells. The A549 cells after 12, 24 and 48 hours incubation to serial dilutions (10-50 μgs) showed decreased cell viability. The cell death was found to be higher after 48 hours. Incubation of cells methylene chloride extract of fungal taxol produced 48% cell viability at 50 μgs after 24 hours and 10% cell viability at 50 μgs after 48 hours (p>0.05). The experiments were carried out in duplicate triplicates.
Cell viability was observed in a dose and time dependent manner in taxol treated cells at increasing concentrations ranging from 10,20,30,40 and 50μgs/ ml. At 24 and 48 hours, less than 50% of cell viability was observed at a concentration of 30 micrograms/ ml. Maximum cytotoxic activity was observed at a concentration of 50μgs/ml at 48 hours.
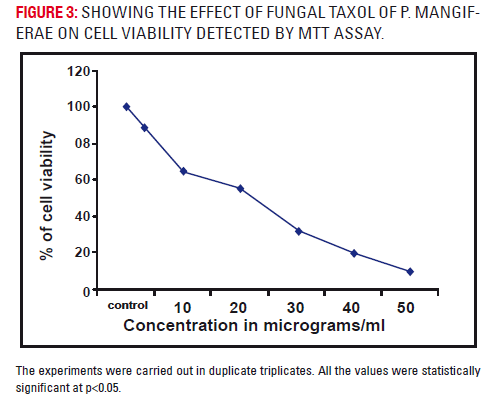
As evident from the figure 4, the cytotoxicity was observed at increasing concentrations i.e.10, 20, 30, 40 and 50μgs (p<0.05). Even at 30 μgs, less than 50% of cell viability is observed while at 50 μgs, only 10% of cell viability was seen.
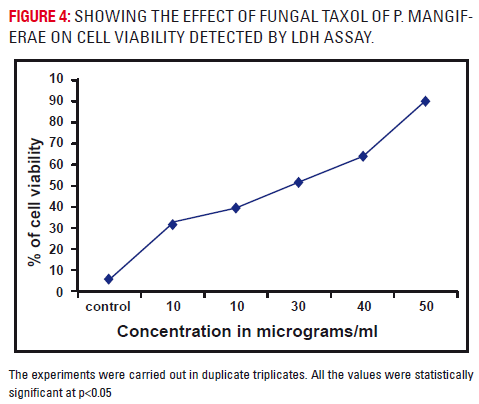
A dose dependent increase in the percentage of LDH leakage was observed. A maximum leakage of LDH was observed at a concentration of 50μgs/ml at 48 hours in taxol treated A549 cells. From the figure 5, the percentage of LDH release was increased at increasing concentrations of methylene chloride extract of fungal taxol which is a direct proportion to cell death. At 50 μgs, the maximum of 90% cell death was observed (p< 0.05) after 48 hours.
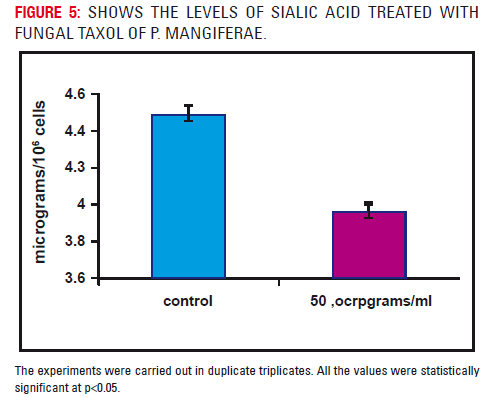
Fig 6 represents the levels of sialic acid in the treated and untreated A-549 cells at a concentration of 50μgs/ml. A marked reduction (p<0.05) in the levels of sialic acid was observed in the taxol treated A549 cells after 48 hours when compared to untreated cells.
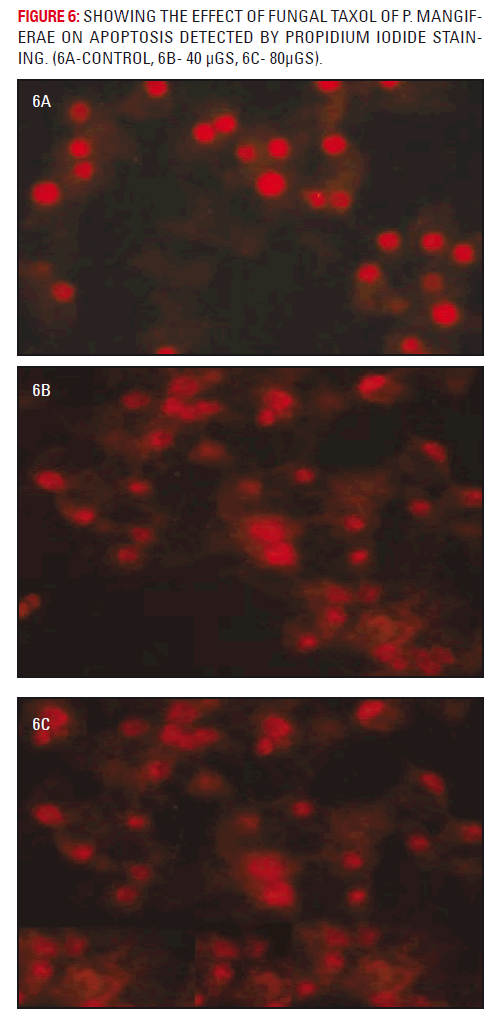
Figure 7 represents the fluorescent microscopic pictures of the methylene chloride extract of fungal taxol treated and untreated A549 cells assessed for apoptosis at 400X.The propidium iodide stained cells at a concentration of 40 and 50 μgs/ml showed the clumping of cells with slight distortion. The highly condensed and fragmented nuclei that are the index of apoptosis were observed at 40 and 50 μgs/ ml (fig 7b and 7c). The untreated A549 cells were shown in plate 7a.
Table 1 shows the levels of DNA, RNA and protein in taxol treated (50 μgs) A 549 and untreated A549 cells. A significant decrease (p<0.05) in the levels of these macromolecules was noted when compared to untreated cells.
| Parameters | Untreated | Treated(50 μgs/ml) |
|---|---|---|
| DNA | 10.86 ± 1.56 | 8.55 ± 1.71* |
| RNA | 14.46 ± 1.75 | 11.88 ±1.06* |
| PROTEIN | 967.07 ±32.86 | 917.14 ± 32.89* |
The experiments were carried out in duplicate triplicates.All the values were statistically significant at *p<0.05, NS-non significant when compared to untreated group (students t-test).Values are expressed as μg/106 cells.
Table 1: showing the effect of fungal taxol of \ on DNA, RNA and protein levels.
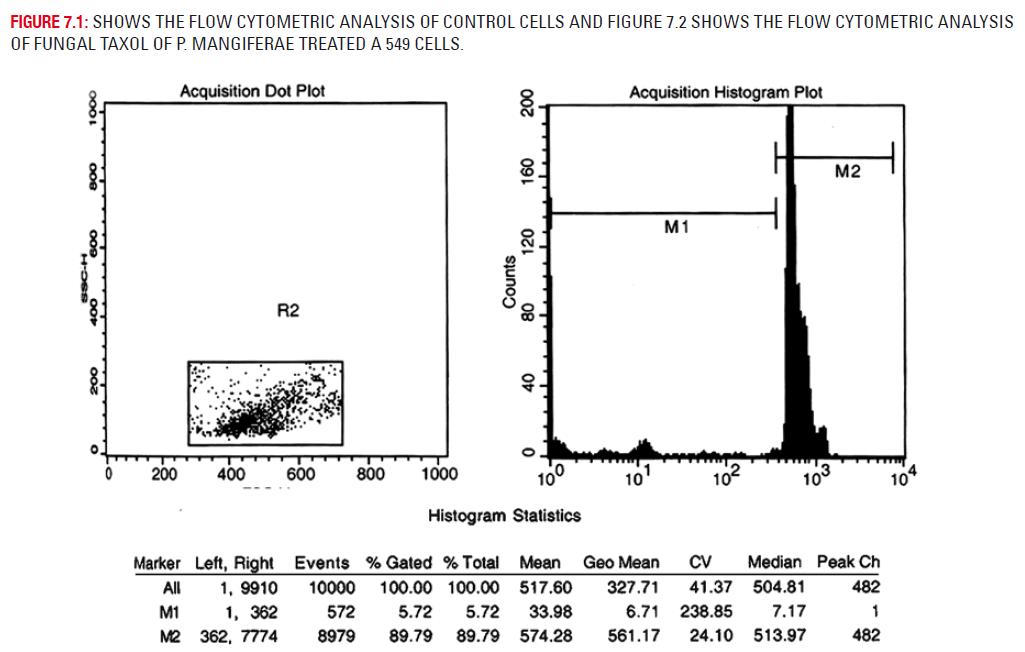
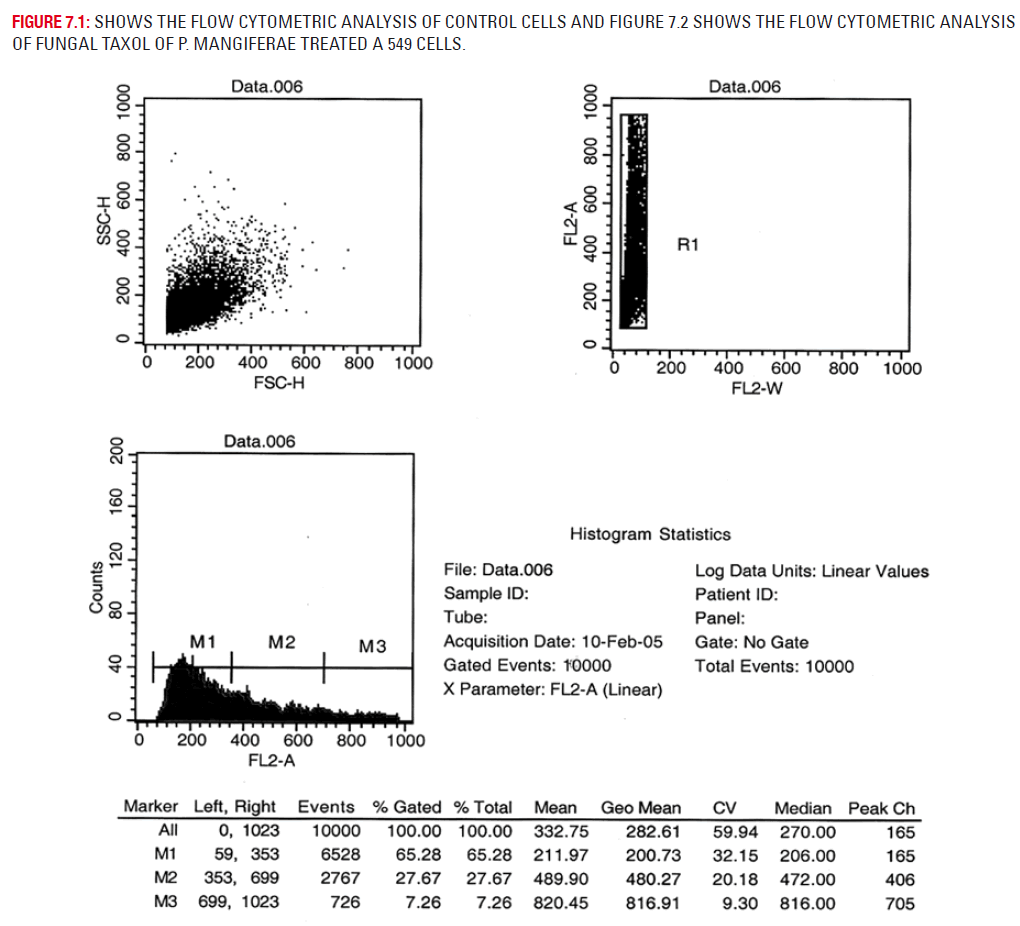
Plate 7.1 depicts the flow cytometric analysis of untreated A 549 cells. Plate 7.2 depicts the drug treated A 549 cells. Flow cytometry analysis for apoptosis study was carried out on the A549 cells after the methylene chloride extract of fungal taxol treatment. It was found that 56.06% of the cells were in the G1 phase, 8.60% in the S phase and 35.78% of cells in the G2 phase in the control cells of A 549 cells. The methylene chloride extract of fungal taxol (5 μgs) treated cells had only 28.64% of the cells in the G1 phase, 4.4% in the S phase and the remaining 50.46% cells in the G2 phase.
Discussion
A number of studies have indicated that significant cell proliferation effect against various cancer lines. In our work, we demonstrated that the methylene chloride extract of fungal taxol extract inhibited the cell proliferation in A549 by inducing apoptotic cell death. The cytotoxic effect was clearly established at increasing concentrations i.e.1, 2, 3, 4 and 5 micrograms. The cytotoxic effect was characterized by disruption of the monolayer. (Kathiravan and Muthumary 2009)
Cancer chemotherapeutic as well as chemopreventive agents exert part of their pharmacological effect by triggering apoptotic cell death or cell cycle transition. Identification of inhibition of apoptosis by several tumor promoters and induction of apoptosis in tumor cells, serves as a predictor of tumor treatment (Kim et al., 1999). Extracts from a broad spectrum of plant species contain substances that possess antitumor activity (Dzham et al., 2002).
The MTT assay, which measures the formazan product at 570 nm, clearly proves the cytotoxicity of the methylene chloride extract of fungal taxol. MTT is cleaved by tetrazolium ring by succinate tetrazolium reductase in active mitochondria. Metabolically active cells cleave MTT and generate a formazan product, which forms purple crystals, and colour developed is directly proportional to cell number (Mossman, 1983).
Based on the results of the cell viability and % of LDH leakage, a concentration of 5μg/ml for further biochemical studies (results not mentioned). LDH leakage is routinely used as an indicator of damage to plasma membrane integrity and in assessing cytotoxic nature of the plant, as dead cells release LDH in to the culture medium (Matsuda et al., 1980). In the present study at 48 hours of incubation a maximum leakage of LDH leakage was observed at a concentration of 5μg/ml, which might be due to the cytotoxic effect of taxol leading to the loss of plasma membrane integrity. Based on the results of cell viability and % LDH release, a concentration of 5μgs/ ml was taken for further studies.
An important component of the cellular response to DNA damage is the inhibition of DNA synthesis. DNA damage could be achieved through in inhibition of a positive regulatory pathway of DNA synthesis and or costimulaton of a negative regulatory pathway. The ability to inhibit DNA synthesis arises from the possibility of interface with DNA replication enzymes and possibility to induce apoptosis (Kajimoto et al., 2002).
Sialic acid is widely distributed in mammals and usually occurs as a terminal component at the non reducing end of carbohydrate side chains of glycoproteins and glycolipids. A increased concentration of sialic acid is observed in the cell surface and sialo groups are separated by some of these cells (Gorczyca et al., 1993). Elevation of sialic acid levels is commonly found in cancer cells (Kastan et al., 1992). Similar results have been observed in the present study with A 549 cells. A decrease in sialic acid levels noticed in taxol treated A549 cells of present study. This could be attributed to the presence of flavanoids in the taxol extract which could mediate the membrane permeabilisation and bring about modifications in the glycoprotein’s components as secondary biochemical responses.
The PI stained cells at a concentration of 5 μgs/ ml showing clumping of cells with slight distortion. A growth inhibitory and apoptosis inducing activity of many flavanoids with the release of cytochrome c is already established (Bauer et al., 1997; May and May, 1999). Evidence indicates that flavanoids may promote apoptotic cell death. The results of the present study reveal that taxol promotes apoptotic mode of cell death in A549 cells which may be attributed to the presence of flavanoids. Detailed investigations are required to establish the actual flavanoids involved and possible potential of taxol in cancer control and chemotherapy.
In the cells undergoing apoptosis, DNA was degraded to fragments of low molecular weight and subsequently leaked out from the cells and the DNA content was stained with a DNA-specific flourochrome, propidium iodide (PI), a special DNA peak (usually called sub-G1 peak) appeared. The G0/G1 population in the methylene chloride extract of fungal taxol treated A 549 cells was increased after 24 hours at 5 μgs/ml. Our cell cycle analysis by flow cytometry showed that there was a prominent increase in the G0/G1 DNA upon methylene chloride extract of fungal taxol treatment. This increase in the G0/ G1 DNA is an indication of the inhibition of DNA replication. The increase of DNA content indicated the retardation of cell cycle, which might have taken place during the G1-S transition phase. The possible mechanism of action would be down regulation of the activity of cyclin E dependent kinase, which plays an essential role for cell cycle progression at the G1/S transition stage. This inhibition of cell-cycle progression might be associated with an altered expression of cell cycle relevant regulator, including p21 and its upstream molecule p53 (Kuo, 1996).
Cell cycle control has been proven to be a major event in ensuring the accurate cell division. Abnormalities of cell cycle regulators have been associated with many carcinogenic processes. The data suggests that methylene chloride extract of fungal taxol could cause a significant accumulation of cells in G0/G1 phase in human lung adenocarcinoma cells (A549) after 24 hours. Our data clearly showed that increase of G0/G1 phase cells was accompanied by decrease of S phase cells. Thus the blockage effect of methylene chloride extract of fungal taxol occurred at G1/S transitions and thus increase of cell numbers in G1 phase was clearly due to decrease of cells in S phase. Much research has showed that the arrest of the cells at the checkpoints of the cell cycle occurs as an event preceding the detection of apoptotic cells (DeAlburuerque et al., 2004).
There may be some factors that affect the relation of test compound effect between in vivo and in vitro, including culture conditions, tumor microenvironment, drug distribution, active metabolites and indirect inhibition effects on tumor proliferation (e.g. angiogenesis). Rather cell culture should be used to illustrate principles, concepts and mechanisms of action that may be active in vivo. If we see it from a drug development point, there are numerous efforts being made to find a method of delivery to achieve the concentration of crude extract in cell cultures.
The idea of study is that to show efficacy in cell cultures first, then start evaluating effects in animals.
Taxol from Pestalotiopsis mangiferae appears to be a promising drug for its future use as an anticancer drug. From our observation, we do agree it is only a preliminary study and it requires a further detailed study.
References
- Chen R, Wei R, Wei L, Chen RL (1995). Lung cancer mortality update and prevalence of smoking among copper miners and smelters. Scand J Work Environ Health 21: 513–516.
- Park JW, Choi YJ, Such SI, Baek WK, suh MH, Jin IN, Min DS, Woo JH, Change JS, Passaniti A, Lee YH, Kwon TK (2001). Bcl-2 overxpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 22:1633-9.
- Courreges MC, Benencia F (2002). In vitro antiphagocytic effect of basil oil on mouse macrophges. Fitoterapia 73: 369-374.
- Steriti R (2002).Nutritional support for chronic myelogenous and other leukemia’s, a review of scientific literature. Altern Med Rev 7(5): 404-409.
- Chopra RN, Nayar SL,Chopra (1956). Indigofera tinctoria,In :Glossary of India Medicinal Plants, CSIR, New Delhi,141.
- Chunekar KC, Bhavaprakasa Nighatu (1993). Chukambha Bharathi academy,Varanasi,India. The Ayurveda Series 28, In; Pandey, GS (ed.),406.
- Kamal R,Mangla M (1990). Flavanoids from I.tinctoria L in vivo and in vitro: Herba polomica 36(12):3-7.
- Lee YS, Han KO, Park CW, Suh S, Shin SW, Yang CH, Jeon TW, Lee ES, Kim KJ, Kim SH, Yoo WK, Kim HJ( 2003). Immunomodulatory effects of aqueous extracted Astragali radix in methotrexate treated mouse spleen cells. J of Ethnopharmacol 84( 2) :193-198.
- Hergartner MO (2000). The biochemistry of apoptosis. Nature 407: 770-776.
- Brown JM, Wooters BG (1999). Apoptosis, P53 and tumor cell sensitivity to anticancer agents. Cancer Res 59:1391-1399.
- S reepriya H, Devaki T, Nayeem M (2001). Protective effects of Indigofera tinctoria L against D – galactosamine and carbon tetra chloride on ‘insitu’ perfused inliver. Indian joumal of physiol. Pharmacol 45 (4): 28-34.
- Mossmann T (1983). Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J.Immunol.Meth 65:55
- Moldeus (1978) : Paracetamol metabolism and toxicity in isolated hepatocytes from rat and mouse. Biochem Pharmacol 27:2859.
- King J (1965a). In: Practical Clinical Enzymology.D.Van.Nostrand Co.,London, pp.83-93.
- Warren L(1959). The thiobarbituric acid assay of sialic acid. J Biol Chem 234:1971-1975.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951).Protein measurement with the folin’s phenol reagent. J Biol Chem 193:265- 276.
- Burton K (1956). A study of the conditions and mechanism of the diphenylamine for the colorimetric estimation of deoxyribonucleic acid.Biochem J 62:315-323.
- R awal UM, Patel US, Rao GN, Desai RR (1977).Clinical and biochemical studieson cateractous human lens III. Quantitative study of protein, RNA and DNA. Arogya J Health Sci 3: 69-72.
- N icotelli I, Pagliacci MC, Grignanif, Ricardi C (1991). A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271-279.
- Kim YS, Jin SH, Lee YH, Kim SI, Par JD (1999).Ginsenoside Rh2 induce apoptosis independently of Bcl 2,Bcl-xL or Bax in C6 Bu-cells. Arch Pharm Res 22 : 448.
- Dzham BB, Das RS, Monteva A (2002). In vitro screening for antitumor activity of chicolodium vulgari L.(Laminaca) extracts.N Biol pharm Bull 25:499.
- M ossmann T. (1983). Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55-63.
- M atsuda M, Osafune M, Nakano E, Kotak T, Sonado T, Hadat T, Watauabe S, Okaochi T, Higashino H, Yamamura X (1980).Lactate dehydrogenase in human renal carcinoma cells. Urol Res 8:201.
- Kajimoto S, Takanashi N, Kajimoto T, Xu M, Cao T, Hasuda Y, Aiuchi T, Nalajo S, Ida Y, Nakaya K(2002). Sophoranone,extracted from a traditional Chinese medicine Shan Dou Geu,induces apoptosis in human leukemia U 937 cells via formation of reactive oxygen species and opening of mitochondrial permeability transistion pores. Int J Can 99, 879.
- Gorczyca W, Gong J, Ardelt B, Tragonos F, Darzynklewiez (1993).The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res 53: 3186.
- Kasten MB, Zhan Q, El-Deiry WS, Carrier F Jacks T, Walsh W, Plunkette BS, Vogelstesn B, Formace AJ Jr (1992). A mammalian cell cycle check point pathway utilizing p53 and GAPD 45 is defective in Atarxia telangictasaia. Cell 71: 581.
- Bauer CH, Visches P, Grunrolz HJ, Reulter W (1997). Glycosyl transferase and glycosidases in morris hepatomas. Cancer Res 37: 1513.
- M ay P, May E (1999). Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 18(53): 7621-36.
- Kuo SM (1996). Antiproliferative potency of structurally distinct dietary flavanoids on human colon cancer cells. Cancer Lett 110 (1-2): 41-48.
- Surh YJ (1999). Molecular mechanism of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428: 305-327.
- DeAlbuquerque GRP, Nakamura CV, De souza W, MoryadoDiuz JA (2004). Differential expression of sialic acid and N-acetyl galactosamine residues on the cell surface of intestinal epithelial cells according to normal or metastatic potential. J Histochem cytochem 52, 629.
- N arender T, Khaliq T, Puri A, Chander R (2006). Antidyslipidemic activity of furano flavanoids isolated from Indigofera tinctoria. Biorg. Med.Chem.Lett 16(1): 3411-3414.
- Kathiravan and Muthumary 2009. Extraction of Taxol, an anticancer drug by Coelomycetous fungi Pestalotiopsis versicolor and Phyllosticta murrayicola. Mycologia Balcanica 6: 69-74.
- Strobel, G.A., W.M. Hess., J.Y. Li, E. Ford, J. Sears, R.S. Sidhu and B. Summerell. 1997. Pestalotiopsis guepinii, a taxol producing endophyte of the Wollemi Pine, Wollemia nobilis. Aust. J. Bot., 45: 1073-1082.
- Strobel, G.A., X. Yang, J. Sears, R. Kramer, R.S. Sidhu, and W.M. Hess. 1996. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiol., 142: 435-440.