Development of Chicken Egg Yolk Antibodies against Streptococcus mitis - Purification and Neutralizing Efficacy
- *Corresponding Author:
Date of Received: 27-04-2012
Date of Modified: 27-04-2012
Date of Accepted: 04-05-2012
Available Online: 15-05-2012
Abstract
Chicken Egg Yolk antibodies (IgY) were raised in 24 week old white leg horn chickens against Streptococcus mitis (MTCC 2696). The chickens received Booster injections of increasing concentrations of antigen to raise the antibody level in egg yolk. The anti-bodies were purified from immunized chicken egg yolk by Poly ethylene Glycol (PEG) and Ammonium sulphate precipitation method and further purified by DEAE cellulose ion exchange column chromatography. High titre of more than 1:10000 antibodies were detected by Indirect antigen capture ELISA at 150th day of observation. IgY con-centration varied in the range of 0.85 – 7.6mg/ml of yolk throughout the immunization period. Growth inhibition assay showed the absence of growth when the specific egg yolk antibodies was added to the Streptococcus mitis culture. Inhibition ELISA shows decrease in absorbance with increasing concentration of IgY. The results indicate that antibodies generated in chicken could be used for diagnosis and therapeutic purposes in case of Streptococcus mitis.
Keywords
S.mitis, Chicken antibodies (IgY), ELISA
Introduction
Streptococci are gram positive cocci arranged in chains. They are widely distributed in nature. Many of them are normal bacterial flora in humans. A few of them are pathogenic. Viridans streptococci are among the first bacteria to colonize the human mouth after birth and generally the predominant colonizing strains are S. mitis and S. oralis. Streptococcus mitis is a Gram-positive commensal bacteria commonly found in the human mouth, throat and nasopharynx. It is one of the most frequent organisms in the oral cavity and is most likely a significant factor in human dental caries due to its acidogenicity and high degree of adhesion to the tooth surface. S. mitis is also one of the most important pathogens in bacterial endocarditis [1]. As other investigators have emphasized, S. mitis can be an important pathogen in children and adults, even though it is more commonly a contaminant of culture media. Considerable diversity exists among the colonizing strains of S. mitis and, in addition, these strains exhibit clonal turnover and replacement. S. mitis contains antigens similar to those of other streptococci. Viridans group streptococci (VGS) are inhabitants of the oral cavity, which is sterile at birth, but at the age of one year, Streptococcus species compose 70% of the cultivable oral flora and Streptococcus salivarius, Streptococcus oralis and Streptococcus mitis are predominant. Different VGS species dominate in different parts of the oral cavity and the gastrointestinal tract and cause different clinical pictures, therefore the identification of the species is important Streptococcus salivarius is found in saliva, on the tongue and buccal surfaces, Streptococcus mitis and Streptococcus mutans are found on the tooth surfaces [2,3].
Antibiotics are often prescribed empirically and penicillin is still considered the drug of choice for the treatment of such diseases. However, the emerging resistance to beta-lactam antibiotics among viridians streptococci may represent a potential clinical problem in the management of therapy. Recent research showed that chicken egg yolk antibodies will act as promising alternative for diagnosis and treatment of S.mutans infection. Passive antibody to S.mutans GBP-B can have a protective effect against cariogenic S.mutans infection and disease [4]. Furthermore, this decrease in infection and disease did not require continuous antibody administration for the duration of the infection period. Therefore, the aim of the present study was to develop chicken egg yolk antibodies against Streptococcus mitis and to evaluate the in vitro activity of egg yolk immunoglobulin (IgY).
Materials and Methods
Experimental animals
Twenty four week old, single comb white leghorn chickens obtained from Poultry Farm and were maintained in our animal facility. They were used in the study for the production of anti Streptococcus mitis antibodies (IgY).
Organism used
Streptococcus mitis was obtained from IMTECH Chandigarh (MTCC 2696) in lyophilized form was used for our present study. The Isolates of Streptococcus mitis were brought to pure culture on Blood agar plates and maintained at 5°C.
Preparation of antigen
Streptococcus mitis was transferred aseptically from blood agar plates to 150ml conical flasks containing sterile Todd Hewitt broth and incubate at 37°C for 24 hours. Following incubation the cells were harvested by centrifugation at 6000 rpm for 15 minutes. The pellet was resuspended in sterile saline and centrifuged again. Then the cells were resuspended in 1ml sterile saline and the cells were heat inactivated by boiling water bath for 20 minutes. After cooling, centrifuge the culture at 6000 rpm for 15 minutes. Bacterial pellet was resuspended in saline. In order to adjust the cells final concentration to 1x108 cells/ml, it was titrated using Mcferlands opacity set.
Development of anti- Streptococcus mitis antibodies in chicken
Streptococcus mitis cells were dissolved separately in 0.9% phosphate buffered saline (PBS) in the concentration of 103 cells/ml. Then antigen was injected intramuscularly at the multiple sites of breast muscles of 24-week-old white leghorn chickens (3 Chickens). Chickens received subsequent booster injections with increasing concentration of antigens (103 cells/ml – 106 cells/ml) at 15 days interval by the same route of administration. Test bleedings were made frequently to check the presence of anti Streptococcus mitis antibodies in the serum. Eggs were collected from day 0 until the end of the experiment and stored at 4°C until testing by the indirect ELISA.
Purification and Characterization of anti- Streptococcus mitis - antibodies from egg yolk
The antibodies were extracted from egg yolk by using Polyethylene and Ammonium sulphate precipitation method [5]. Briefly, the egg yolk was separated from white, washed with distilled water to remove as much albumin as possible and rolled on a paper towel to remove adhering egg white. The membrane was punctured and the yolk without the membrane was allowed to flow into a graduated cylinder. An equal amount of buffer S (10 mM phosphate, 100mM Nacl, pH 7.5, containing 0.01% sodium azide) was added to the yolk and stirred. To this mixture 10.5% PEG 8000 in buffer S was added to a final concentration of 3.5%. The mixture was stirred for 30 minutes at room temperature and centrifuged at 11000 rpm for 20 minutes. The supernatent was filtered through double-layered cheesecloth. The 42% PEG in buffer S was added to make final concentration of 12% PEG. The mixture was stirred thoroughly and centrifuged at 11000 rpm for 20 minutes. The pellet was redissolved in buffer S to the original yolk volume and an equal volume of 4M Ammonium sulphate (pH 7) was added and the precipitate was centrifuged at 11,000 rpm for 20 minutes, the pellet was redissolved in 1ml of buffer S (without NaCl) over night. Then the content was desalted by dialysis process. The crude fraction of IgY thus obtained was further purified by DEAE cellulose ion exchange column chromatography. The IgY fraction was then concentrated with Poly Vinyl Pyrolidone (PVP) at room temperature. The protein content of the purified IgY fraction was determined by the method described by Lowry et al. (1951) [6]. The purity of Chicken egg yolk antibodies was checked by SDS-PAGE [7]. The Chicken egg yolk antibodies were also checked on Native Gel (PAGE).
Determination of antibody titer by Indirect ELISA
The antibody titer of the antibodies generated against Streptococcus mitis was determined by indirect antigen capture ELISA [8]. Nunc polysorp ELISA plates were coated with Streptococcus mitis antigens using coating buffer (0.05M carbonatebicarbonate buffer, pH 9.6) and incubated at 4°C overnight. After coating plates were washed with PBST for 3 times. The empty sites blocked by 1% BSA (200 μl/well) and incubated at 37°C for 1 hour. Plates were subsequently washed and incubated with anti- Streptococcus mitis - antibodies (100 μl/well). PBST and preimmune sera served as controls. Wells were washed thrice with PBST and 100 μl of diluted (1:1000) rabbit antichicken immunoglobulin coupled to Horse Radish Peroxidase (Genei Pvt Ltd, Bangalore) was added and incubated. Then the plates were washed and 100 μl of freshly prepared substrate solution (4 mg of O-phenylene diamine dissolved in 10 ml of 50 mM citrate buffer, pH 5.0 containing 10 μl of 30% hydrogen peroxide) was added. The plates were allowed to stand at room temperature in dark for 20 minutes. The reaction was stopped by adding 50 μl of 4N sulphuric acid and plates were read at 490 nm in an ELISA reader. All samples were tested in triplicates.
Growth Inhibition Assay and Microscopic Slide Agglutination test
Growth Inhibition Assay was carried out by novel method. This experiment is to check whether the anti- Streptococcus mitis IgY could inhibit Streptococcus mitis growth in liquid medium. Streptococcus mitis was inoculated into 5ml Todd hewitt Broth (THB), and into another 5 sets of 5 ml of THB containing increasing concentrations of chicken egg yolk antibodies (5μg/ml of IgY - 25μg/ml of IgY) and incubated overnight at 37°C. PBS alone served as negative control and 100 μg/ml of penicillin was the positive control. After incubation the contents were subcultured onto Blood agar and incubated overnight at 37°C. The Tubes also checked for OD and Turbidity method to check the growth inhibition of Streptococcus mitis by chicken egg yolk antibodies (IgY). Antibacterial effect IgY against Streptococcus mitis was tested and identified by Microscopic Slide Agglutination Test (MSAT).
Inhibition ELISA
Various concentrations of chicken egg yolk anti bodies (IgY) raised against Streptococcus mitis (MTCC 2696) was pre-incubated with overnight cultures of Streptococcus mitis (2.5 x 106 cells/ml), and added to microtitre plate wells previously coated with Streptococcus mitis antigen. After washing steps the bound antibodies were reacted with rabbit antichicken immunoglobulin coupled to Horse Radish Peroxidase and developed with OPD and H2O2. The reaction was stopped by adding 50 μl of 4N sulphuric acid and plates were read at 490 nm in an ELISA reader.
Results
Generation of Streptococcus mitis antibodies in chicken
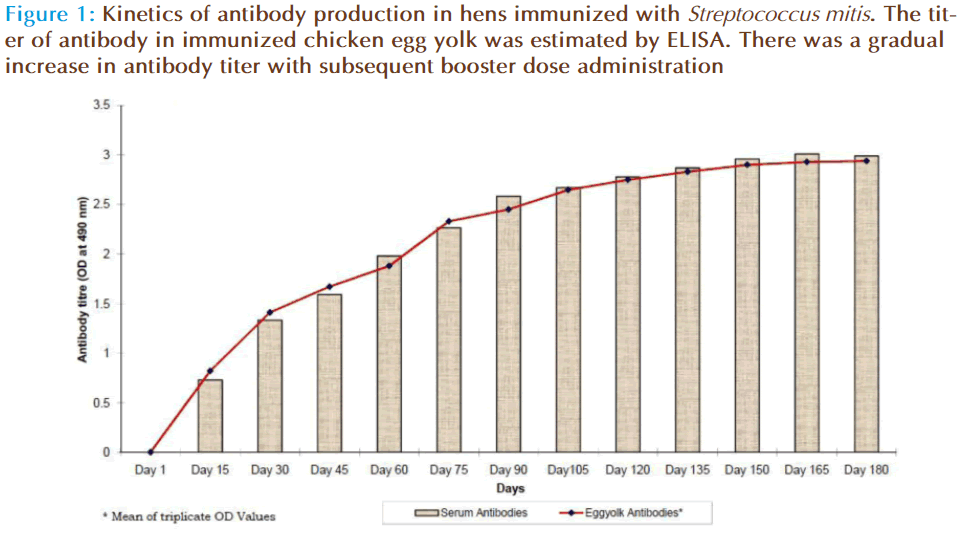
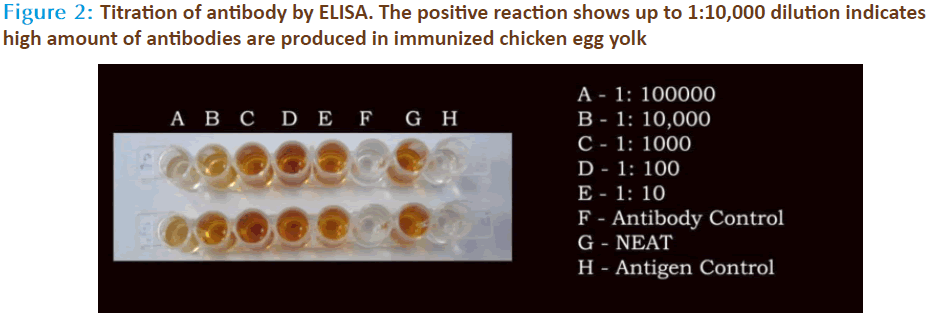
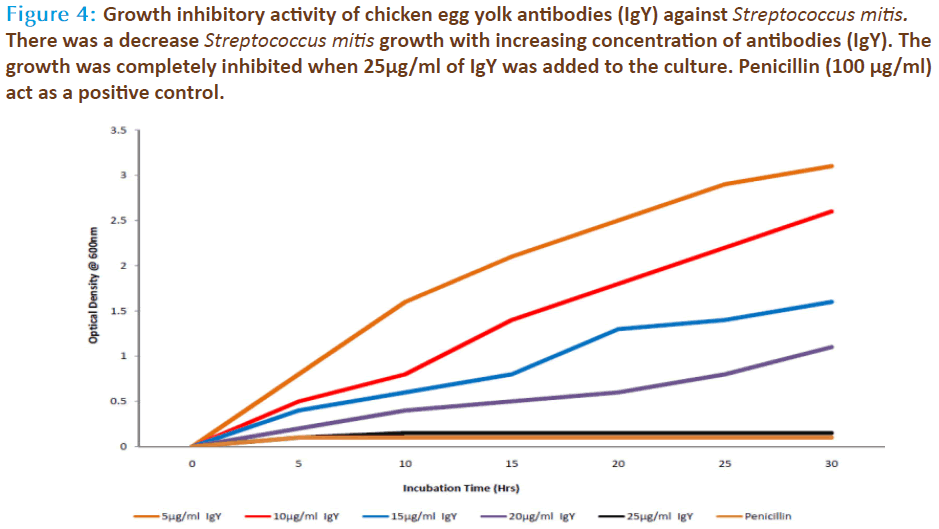
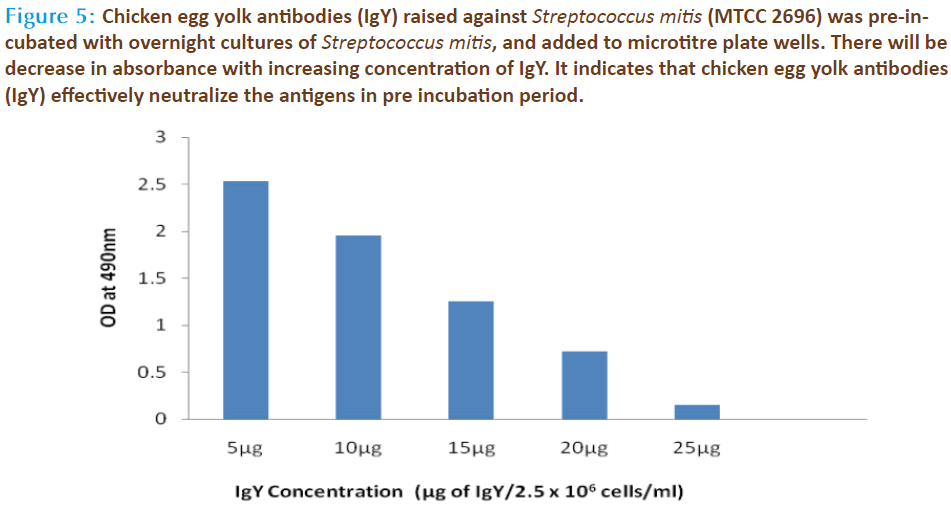
The source for anti-Streptococcus mitis was obtained through chicken serum and in the same chicken’s egg yolk. The preimmune sera and hyperimmune sera were collected at specific time intervals during and after various immunization schedules. The egg yolk antibodies was purified by the method of polyethylene glycol and ammonium sulphate precipitation method from immunized chicken egg yolks and was further purified by DEAE cellulose ion exchange column chromatography. Agglutinating antibodies were observed in both serum as well as egg yolk’s of immunized white leghorn chickens. The antibody concentration of such purified fraction after each booster doses was detected by protein estimation. The IgY concentration in the egg yolk significantly increased when the chickens received booster injections at regular intervals of time. In the present study, IgY concentration varied in the range of 0.85 – 7.6mg/ml of yolk throughout the immunization period. The titre of antibodies in the immunized chicken egg yolk was carried out by ELISA. Indirect antigen capture assay (IACA) showed that the antibodies were generated in chicken against Streptococcus mitis antigens. High peak titre of 1:10000 monovalent antibody were observed during 150th day of observation (Fig 1 & 2). The antibody levels of the immunized animals were significantly higher than those of the controls. This indicates that the production of specific IgY can be efficiently elicited in chickens using simple protocols of immunization and extraction. A single protein band of high molecular weight (180KDa) observed in electrophorectic analysis showed the purity of IgY (Fig 3). In native gel we observed florescent band emitted under UV. The activity of anti-Streptococcus mitis IgY was shown by the absence of growth when the specific egg yolk antibodies were added to the Streptococcus mitis culture. There was a decrease in Streptococcus mitis growth with increasing concentration of antibodies (IgY). The growth was completely inhibited when 25μg/ml of IgY was added to the culture (Fig 4). In inhibition ELISA there will be decrease in absorbance with increasing concentration of IgY. It indicates that chicken egg yolk antibodies (IgY) effectively neutralize the antigens in pre incubation period (Fig 5).
Figure 4: Growth inhibitory activity of chicken egg yolk antibodies (IgY) against Streptococcus mitis. There was a decrease Streptococcus mitis growth with increasing concentration of antibodies (IgY). The growth was completely inhibited when 25μg/ml of IgY was added to the culture. Penicillin (100 μg/ml) act as a positive control.
Figure 5: Chicken egg yolk antibodies (IgY) raised against Streptococcus mitis (MTCC 2696) was pre-incubated with overnight cultures of Streptococcus mitis, and added to microtitre plate wells. There will be decrease in absorbance with increasing concentration of IgY. It indicates that chicken egg yolk antibodies (IgY) effectively neutralize the antigens in pre incubation period.
Discussion
Dental plaque is a complex, multispecies biofilm that forms on oral surfaces in which bacteria are embedded in bacterial and salivary polymers. Many species and strains have been identified in dental plaque; some of these organisms are now recognized as early colonizers, and others are considered cariogenic organisms or periodontopathogens. However, no real ecological role has been defined for most strains and species in dental plaque, and it is possible that these organisms should be considered part of the normal, indigenous, healthy microflora in the oral cavity. S. mitis, one of the more common species of viridans streptococci, has generally been minimally pathogenic and relatively avirulent. Even when it causes endovascular infection such as subacute bacterial endocarditis, the affected patients are often not severely ill. When isolated from upper respiratory samples, it is often dismissed as a nonpathogenic commersal. Some aggressive strains have been reported to cause sepsis, acute respiratory distress syndrome, and shock in neutropenic hosts. Viridans streptococci have become increasingly resistant to antibiotics including penicillin, cephalosporin, erythromycin, and tetracycline. S. mitis is more resistant to antibiotics than other viridans streptococci. Viridans streptococci can transfer their resistant genes to more pathogenic pneumococci and group A streptococci. Among the viridans streptococci, Streptococcus mitis has been considered the species with the highest level of resistance to penicillin. Recent research showed that chicken egg yolk antibodies will act as promising alternative for mammalian antibodies. Passive antibody to S. mutans GBP-B can have a protective effect against cariogenic S.mutans infection and disease [4]. Furthermore, this decrease in infection and disease did not require continuous antibody administration for the duration of the infection period. Chicken antibodies were upto six times as potent as horse antibodies [9]. Equally important, the antibodies in egg yolk are highly concentrated purer than those in mammalian blood. Chicken egg yolk antibodies are cheaper, simple to produce in larger amounts, whereas other polyclonal and monoclonal antibodies are expensive and difficult to produce [10,11].
Hence, an attempt has been made to generate Streptococcus mitis antibodies in chicken as well as in the yolk of the immunized chicken. In the present investigation also we could observe that high amount of antibodies against Streptococcus mitis can be generated using white leghorn chickens.
Advantages of chicken antibodies
The antibodies against Streptococcus mitis antigen from immunized chicken egg-yolks were determined at periodic intervals. The agglutinating type of antibodies were observed after 1 month of immunization. The concentration of protein in egg yolk was estimated as an indicator of the level of agglutinating antibodies. The maximal protein concentraion of 5.9 mg/ml of egg- yolk was observed after 70th day. Additionally, the yolk of egg laid by immunized chicken has been recognized as an excellent source of polyclonal antibodies for over a decade. A single egg contains as much polyclonal antibodies as an average bleed from a rabbit would yield. Typically each egg may contain 90-100 mg of IgY, of which 10% may be against specific antigen [5]. Our study also indicates that almost a similar yield of IgY antibodies per egg were obtained.
The present study showed that chicken hyperimmune serum and egg yolk antibodies can be used for the treatment of Streptococcus mitis infections. Chicken antibodies can be prepared in large scale since chicken IgY is a continues source from immunized chickens, which makes it possible to produce in large amount. Hence chicken egg yolk antibodies may serve as an alternative source to neutralize the Streptococcus mitis. Much more studies are required to evaluate the potency of Streptococcus mitis antibodies. Chicken egg yolk antibodies will play an increasing role in research, diagnostics and immunotherapy in future.
References
- Lars Linder, Carita Andersson, Marie-Louise Sund, et al. Protoplast Formation and Localization of Enzymes in Streptococcus mitis. Infection and Immunity. 1983; 40(3): 1146-1154.
- Katherine A. Wirth, George H. Bowden, Dorothy A. Richmond, et al. Antibody binding to Streptococcus mitis and Streptococcus oralis cell fractions. Arch Oral Biol. 2008; 53(2): 141–149.
- Jens Kreth, Justin Merritt, Fengxia Qi. Bacterial and Host Interactions of Oral Streptococci. DNA Cell Biol. 2009; 28(8): 397–403.
- Smith D J, King WF, Ronald Godiska. Passive transfer of Immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infection and Immunity. 2001; 5: 3135 – 3142.
- Polson A, Von Wechmar MB, Van Regenmortel M H V. Isolation of viral IgYantibodies from yolks of immunized hens. Immunological Communications. 1980 ; 9: 475 - 493.
- Lowry O H, Rosebrough N J, Farr A L, et al. Protein measurement with the folin-phenol reagent. J.Biol.Chem. 1951; 193: 265 - 275.
- Laemmeli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680 - 685.
- Voller A, Ann Bartlett, Bidwell D E. The Detection of Viruses by Enzyme- Linked Immunosorbent Assay (ELISA). J. gen. Virol. 1976; 33: 165 - 167.
- Thalley B S, Carroll S B. Rattlesnake and scorpion antivenin from the egg yolks of immunized hens. Biotechnology. 1990; 8: 934 - 938.
- Jensenics J C, Andersen I, Hau J, et al. Eggs: conveniently packaged antibodies, methods for purification of yolk IgY. J. Immounol. Methods. 1981; 46: 63 - 68.
- Jann Hau, Hendriksen C F M. Refinement of Polyclonal Antibody Production by Combining Oral Immunization of Chickens with Harvest of Antibodies from the Egg Yolk. ILAR. 2005; 46: 294 – 299.