Current Regulatory Challenges and Approaches in Registering In-Vitro Diagnostics (IVD’s) in India
Citation: Saminathan J, Patel R. Current Regulatory Challenges and Approaches in Registering In-Vitro Diagnostics (IVD’s) in India. J Basic Clin Pharma 2017;8:S125-131.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
The quality of medical devices is governed by regulatory authorities of the respective countries. The regulations are intended to protect the risks associated with design, manufacture and packaging of medical devices. India is one of the top twenty markets for medical devices in the world and the fourth largest market in Asia. The National Medical Device Policy 2015 is framed out by the Government of India to strengthen the medical device market and decreasing dependence on importing the medical devices. In vitro diagnostic (IVD’s) are used for the invitro examination of specimens derived from the human body solely or principally to diagnose the problems. The Indian medical device sector is about USD 4.9 billion in which of 53% are IVD’s. The in-vitro diagnostic advisory committee (IVDAC) is prepared by Government of India to give advice in matters related to IVD’s to DCGI and approve products of IVD’s. The Schedule M-III and M-IV of D and C act 1940 and rules 1945 are led down, which provides information on quality management system and GMP practices and requirements of premises, equipment and plant for In vitro diagnostic reagents and kits respectively. Registration procedure of IVD’s, common submission format for registration, labelling requirements, regulatory scenario and recommendations to the government has been mentioned.
Keywords
In vitro diagnostics, regulatory affairs, registration, labelling, regulatory scenario and recommendation
Introduction
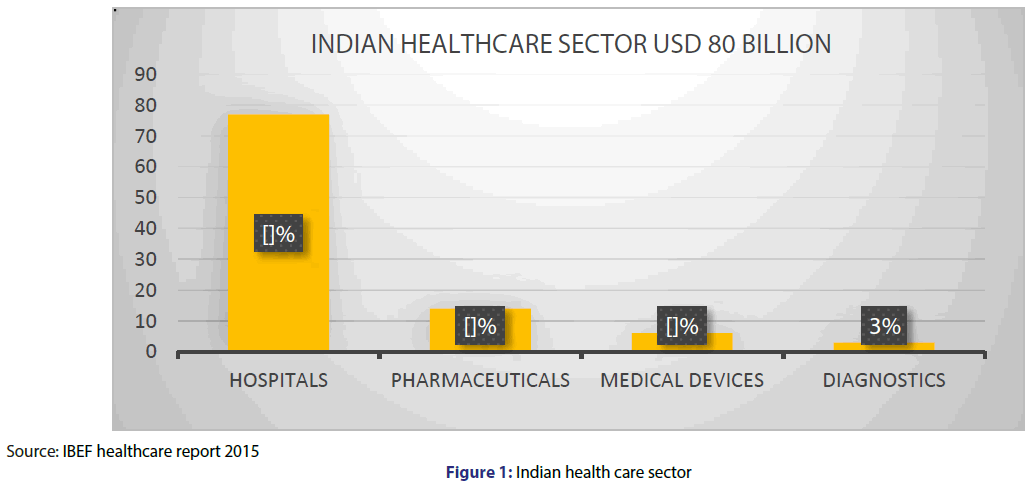
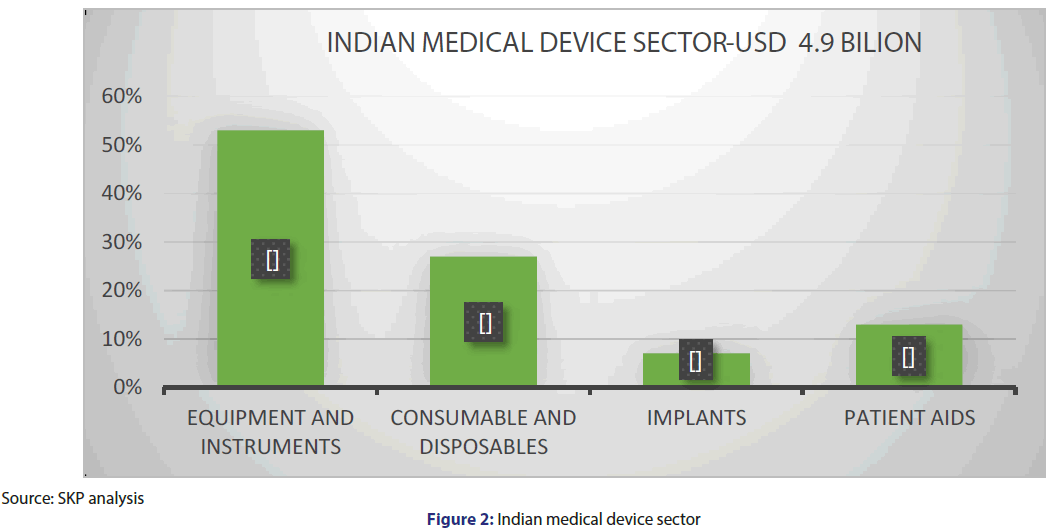
With the increases in opportunities, Medical Device Companies has to face more and more regulations and reach up to its standards. The industry is turning technology intensive by producing high quality and cost-effective medical devices. On the other hand, the present Indian medical device industry landscape is primarily import driven with imports contributing close to 75% [1] of the market. Every day more than 50,000 medical devices are used in the healthcare sector, so patient’s safety is important to maintain by the regulatory authorities. [2] So, medical devices need to be regulated. Indian healthcare sector consists of USD 80 million in which medical devices consist of 6% market and 3% of In-Vitro diagnostic devices as shown in Figure 1. Indian medical device sector is of USD 4.9 billion in which 53% is IVD’s (equipment and instruments), 27% is consumable and disposables, 13% is patient aids, 7% is implants [3,4] shown in Figure 2.
According to National medical device policy 2015, the global medical device market is $220 billion and India is smaller as compared to rest of the countries having 20th position globally. NMDA (National Medical Device Authority) is responsible for managing medical device bodies and develop benchmark in the international market. The NMDA shall provide a single window mechanism for the industry to make country self-reliable. [5,6] The government on the recommendation of NMDA will provide budgetary resources. The authority will designate “Centres for Excellence” for supporting product development and validation and set up a skill development committee. They will separate the pricing for the medical device so that they are entered into the separate list of commodities under the Essential commodities act.
The Indian medical device market is worth of about USD 3 million and contributes to 6% of India’s USD 40 billion healthcare industry. [7] India is one of the top twenty markets for medical devices in the world and is the fourth largest market in Asia but despite it has to face many challenges i.e.,
1. Achieve all the regulatory standards
2. The company has to merge with a government to promote its product
3. Lack of talent
4. Lack of provided tax incentives.
5. Import dependency is high
6. Lack of Research and development fund
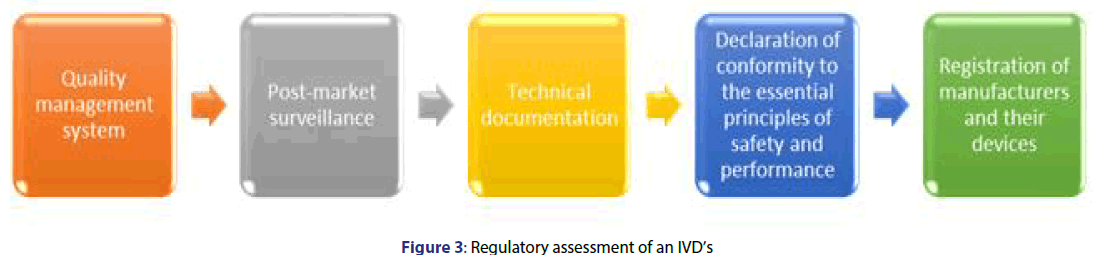
According to GHTF (Global Harmonization Task Force), In-Vitro medical devices (IVD’s) whether used alone or in combination are used for the in-vitro examination of specimens derived from the human body solely or principally to diagnostic, monitoring[8,9] or compatibility purposes shown in Figure 3. They may include reagents, calibrators, control materials, kits etc.
Classification of IVD’s medical devices
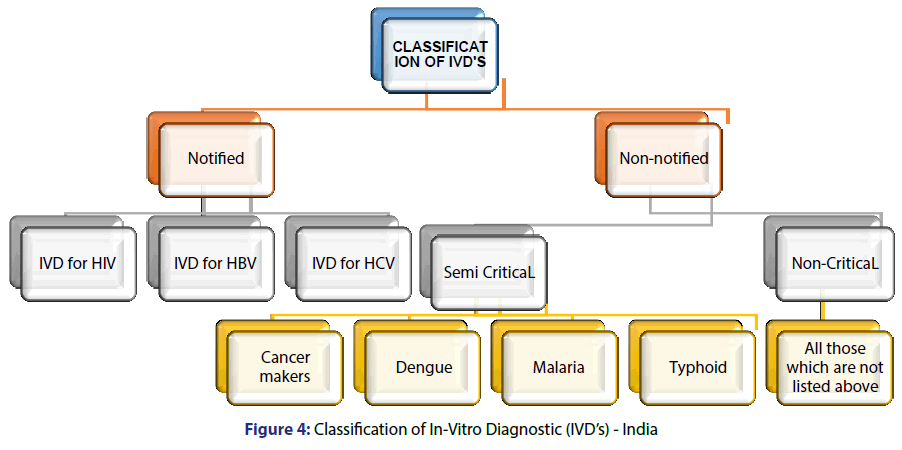
The GHTF has proposed the classification system for IVD’s medical devices based on the risk involved as shown in Table 1. The classification of IVD medical devices is based on following criteria with Annexure IX of schedule M-III provided by the Drug technical advisory board (DTAB) shown in Figure 4.
| CLASS | RISK LEVEL | DEVICE EXAMPLE |
|---|---|---|
| A | Low individual, Risk and low public health risk | Clinical chemistry analyzer, prepared selective culture media |
| B | Moderate individual risk and low public health risk | Vit B12, Pregnancy self-testing, anti-nuclear antibody, urine test strips |
| C | High individual risk and moderate public health risk | Blood glucose self-testing, HLA typing, PSA screening , rubella |
| D | High individual risk and high public health risk | HIV blood donor screening, HIV blood diagnostic |
Table 1: Classification system for ivd’s medical devices based on risk
For registration and import of any IVD’s in India, the company has to follow D and C act 1940 and rules 1945 which is governed by Drug controller general of India (DCGI), CDSCO, Directorate General of Health Services, GOI. In case of Notified IVD’s shown in Table 2. For registration form 41 and import licence in form 10 are required as per D and C act 1940 and rules 1945 24-A (form and manner of applicant for registration certificate), 25-B (registration certificate for import of drugs manufactured by one manufacturer), 27-A (Grant of registration certificate), 29-A (suspension and cancellation of registration certificate) and 28-A (Duration of registration certificate) of Drugs and cosmetic act proves the information of grant of registration certificate. While in the case of Non-notified IVD’s they are regulated [10] under sub clause (i) of clause (b) of section 3 of D and C act 1940 and rules 1945.
| S.no | Name of the Device | Notification Number | Date of Notification |
|---|---|---|---|
| 1. | Disposable Hypodermic Syringes | GSR 365 (E) | 17-03-1989 |
| 2. | Disposable Hypodermic Needles | GSR 365 (E) | 17-03-1989 |
| 3. | Disposable Perfusion Sets | GSR 365 (E) | 17-03-1989 |
| 4. | In-vitro Diagnostic Devices for HIV, HBsAg and HCV | GSR 601(E) | 27-08-2002 |
| 5. | Cardiac Stents | S.O. 1468 (E) | 06-10-2005 |
| 6. | Drug Eluting Stents | S.O. 1468 (E) | 06-10-2005 |
| 7. | Catheters | S.O. 1468 (E) | 06-10-2005 |
| 8. | Intra Ocular Lenses | S.O. 1468 (E) | 06-10-2005 |
| 9. | I.V. Cannulae | S.O. 1468 (E) | 06-10-2005 |
| 10. | Bone Cements | S.O. 1468 (E) | 06-10-2005 |
| 11. | Heart Valves | S.O. 1468 (E) | 06-10-2005 |
| 12. | Scalp Vein Set | S.O. 1468 (E) | 06-10-2005 |
Table 2: Notified medical devices under drugs and cosmetics act
Notified medical devices
Immuno Diagnostic Kit Laboratory is notified as Central Drugs Laboratory (CDL) by Government of India vide Gazette No. G.F.R 601 E, dated 27th August 2002 for In-Vitro diagnostic devices for HIV, HCV, and HBsAg. The lab has a Quality Management System in Place and is NABL accredited in accordance with the standard ISO/IEC 17025:2005 in the field of Immunodiagnostic kits vide certificate no. T-2011 dated 05.12.2013 for Biological Testing. The Laboratory has been conducting Quality evaluations of indigenously manufactured and imported kits (Rapid, ELISA, CLIA, ELFA, Confirmatory, Ag/Ab and Combo kits) for HIV, HCV, HBV and Syphilis. For import of medical device, the manufacturing site and IVD’s are required to be registered with an Indian drug regulatory agency like CDSCO. [11] All devices which fall under regulation has to submit clinical trial study reports to prove its performance in comparison with other products present in the market. The Government of India (GOI) is responsible for regulating medical devices and maintains their safety, efficacy and quality. The import, manufacture, sale and distribution of medical devices are regulated under the provisions of the D and C act 1940 and rules 1945 and only notified medical devices are regulated in India. Manufacturing for sale of In-vitro Diagnostic Devices is regulated by the concerned State Drug Licensing Authority (SLA) only. A single application has to be made to SLA for obtaining the manufacturing license.
(a) In-Vitro Diagnostic Devices for HIV: The Human immunodeficiency virus (HIV) is a lentivirus (a subgroup of retrovirus) that causes HIV infection and over time acquired immunodeficiency syndrome (AIDS). AIDS is a disease which causes progressive failure of the immune system of humans. If kept untreated the person may survive for 9-11 months only.
(b) In-Vitro Diagnostic Devices for HBV: The Hepatitis B virus (HBV) is a part of Hepadnaviridae family. It is a small DNA virus similar to retroviruses. It is classified into 8 genotypes from A to H. HBV replicates into RNA intermediate and can integrate into the host genome.
(c) In-Vitro Diagnostic Devices for HCV: Hepatitis C virus is a bloodborne disease which causes both acute and chronic infection. The vaccines are still not available.
(d) In-Vitro blood grouping sera: WHO is involved in this diagnostic process since 1950s and numerous international reference preparations are developed to curb blood typing reagents. Blood transfusion involves In-Vitro diagnostic testing for detecting blood groups and it is performed for decades. After the review in 1988, the Expert Committee on Biological standardization (ECBS) has pronounced the replacement of anti-D, complete, anti-D, and incomplete as well as anti-A and anti-B would be a priority.
Non-notified medical devices
All those kits and reagents which are not in above list are covered under Non Notified IVD products.
Schedule M-III of drugs and cosmetic act gives the detail quality management system for Notified medical devices and In-Vitro medical devices which includes
► Documented statements of a quality policy and quality objectives
► Quality manual
► Documented procedure required by this schedule
► Documents needed by the manufacturer to ensure the effective planning, operation, control of its processes
► Records required by this schedule.
Schedule M-IV of D and C act 1940 and rules 1945 is led down which provides information on Good manufacturing practices and requirements of premises, equipment and plant for In-Vitro diagnostic reagents and kits which include:
* Location and surroundings: The factory of IVD’s should be located in the industrial area and measures should be taken to avoid the risk of contamination.
* Building and premises: The buildings and premises should be made to suit the manufacturing and production of IVD’s.
* Water system: there should be validated water system for the movement from source to destination.
* Disposal of water: The disposal of effluents from factory shall be according to Environment pollution control board.
* Warehouse area: Adequate areas should be prepared to store different categories of materials and products like raw material, finished products
* Production area: Production area should allow production of uniflow with logical sequence.
* Ancillary area: Rest and refreshment areas should be separate from production or manufacturing areas.
* Quality control area: This area should be designed appropriately and avoid cross contamination and mix-ups.
* Health, clothing and sanitation of workers: every worker should undergo periodic health check-ups including eye test and shall be free from tuberculosis etc.
Registration procedure of IVD’s devices
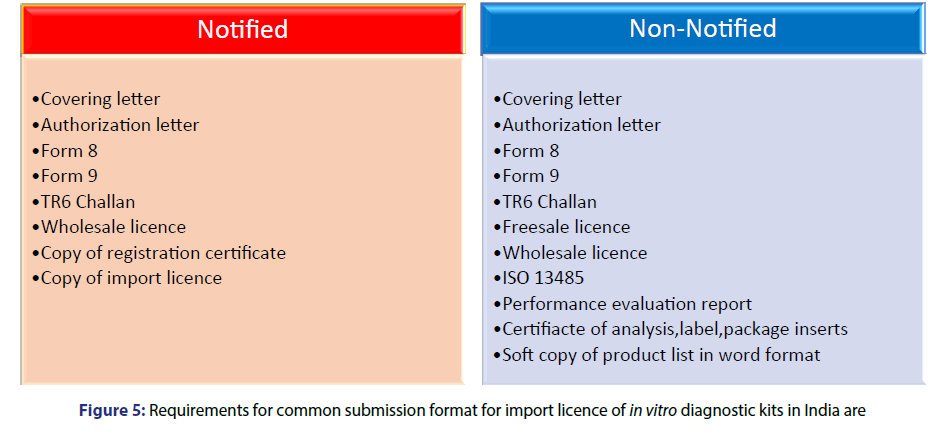
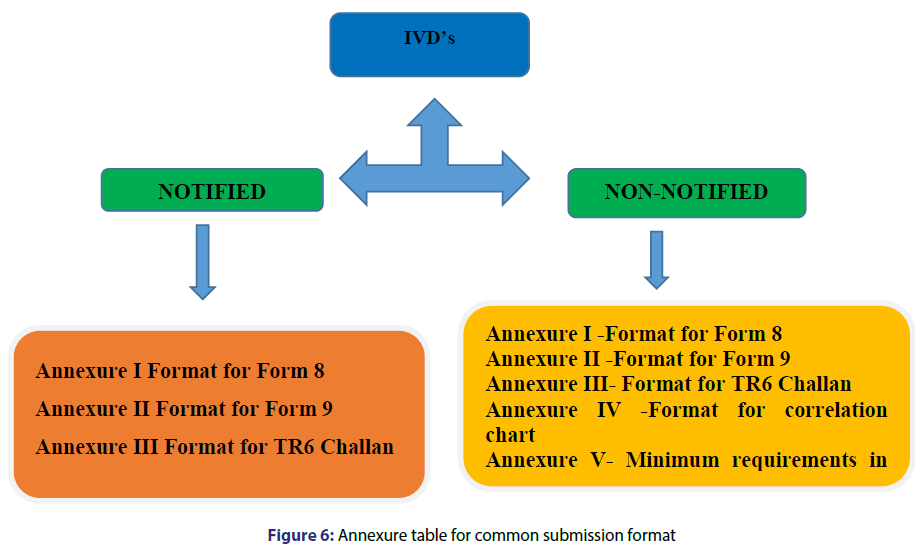
For registration of IVD’s in India first of all the company has to determine the category of the product then Indian agent has to be there who will proceed the procedure of registration of the product. The company further has to file an application for issue of registration certificate i.e., form 40 along with the required documents which are enclosed in Annexure-I of D and C act 1940 and rules 1945 afterwards the registration certificate is issued in form 41. Requirements for common submission format for registration of In-Vitro diagnostic devices in India are shown in Figures 5 and 6.
a) Covering letter: In the covering letter, the applicant has to specify whether they are applying for registration or re-registration of the product. The list of documents that they are submitting with index should also be mentioned. This letter should be signed and stamped by the authorized signatory.
b) Authorization letter: It is issued by the director of the company of the applicant that informs about the person in charge for signing important documents.
c) Form 40: It is the application for issue of a registration certificate for import of drugs into India under the D and C act 1940 and rules 1945 which is enclosed in Annexure-I of D and C act 1940 and rules 1945.
d) TR6 challan: The fees for a particular device should be submitted to the notified branch of Bank of Baroda and it is enclosed in annexure –II of D and C act 1940 and rules 1945.
e) Power of attorney: The authorization by a manufacturer to his Indian agent shall be documented as the power of attorney which enclosed in Annexure –III of D and C act 1940 and rules 1945.
f) Wholesale license- The application for a wholesale license should be applied according to rule 20B and 21B and for renewal rule 21C of D and C act 1940 and rules 1945 should be followed.
g) ISO 13485 certificate: The ISO is International Organization for Standardization which represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices. It should be duly signed and stamped by Indian embassy with respect to the manufacturing site.
h) Performance Evaluation Report: The product should be approved from National institute of Biologicals, Noida for 3 consecutive batches. It also includes batch number, testing procedure, manufacture’s name, etc.
i) Schedule D-I: plant/site master file are enclosed in annexure V of D and C act 1940 and rules 1945.
j) Schedule D-II: schedule DII includes device master file which is enclosed in annexure VI of D and C act 1940 and rules 1945.
The registration certificate is valid for three years from the date of issue unless it is cancelled. If the applicant fails to comply with the conditions of the registration certificate the licensing authority after giving time for an explanation can cancel or suspend the certificate. The separate license is required for each manufacturing location which will be approved by Drug controller general of India. The registration procedure may take 4-9 months for completion. The In-vitro diagnostic device advisory committee (IVDAC) is prepared by the government of India to give advice in matters related to IVD’s and approval of products related to IVD’s to DCGI. While approving the product the IVDAC has to examine the essentiality, desirability and effectiveness of the new diagnostic device. The global trails can also be examined by the committee.
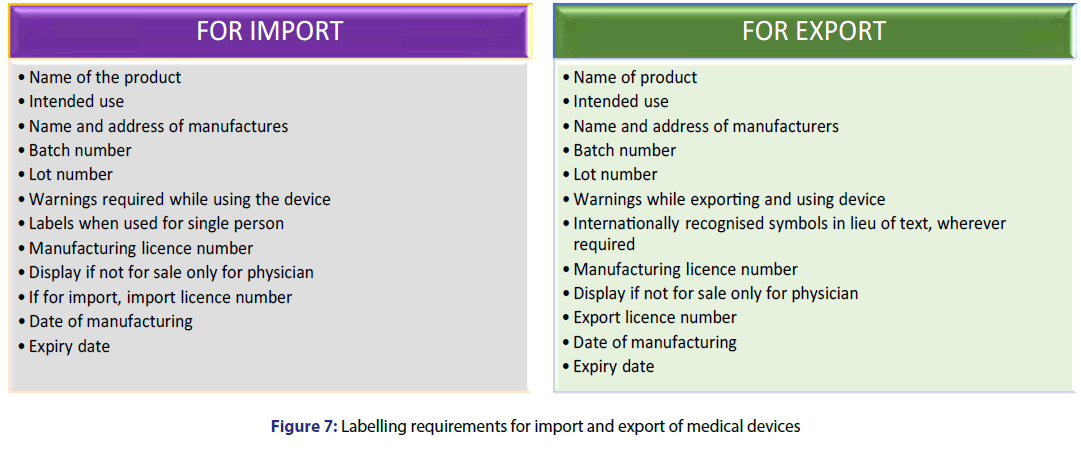
Labelling requirements for import and export of medical devices
The Labelling requirements (12) for Import and Export of medical devices shall be printed in indelible ink on the label or sticker on the shelf pack of the medical device or on the outside of such medical device and on every outer covering in which the medical device is packed shown in Figure 7. Under Rule 109A of D and C Act.
The label, in case of imported devices, with the approval of the licensing authority mentioned in rule 21, the import licence number, name and address of the importer and address of the actual manufacturing premises, date of manufacture, The label may bear symbols recognised by the Bureau of Indian Standards or International Organisation for Standardisation (ISO) in lieu of text and the device safety is not compromised by a lack of understanding on the part of the user in case the meaning of the symbol is not obvious to the device user.
Regulatory scenario
The Indian medical devices market [12,13] will benefit from the expansion of health insurance. [14] Approximately 72% of medical devices in India are imported. A World Bank estimation reveals that India spends only 1% of its GDP on public health which includes accounting for recurrent and capital spending from government budgets, external borrowings, grants and social health insurance. In India, healthcare is a major investment business which is increasing with the population and its higher demand and expectation [15,16] towards it. India’s relatively low per capital expenditure on healthcare up to Date, India’s market for medical devices is in the world’s top twenty in 2007 India’s medical Equipment market was estimated at about $1.56 billion. The market is expected to grow about 8% annually and approach $2.3 billion by 2012 (source: Espicom Business Intelligence). [17-20]
Along with the drugs, medical devices market is growing fast crossing all the hindrance that occurs due to inadequate quality standards setting, time delays and other related hassles, new product development. The import, manufacturing, distribution and sale of medical devices in India are overseen by the D and C act 1940 and rules 1945. Controls and inspections are carried out by the CDSCO, state drug controllers and central/state laboratories. CLAA (Central Licenses Approving Authority) - Branch of CDSCO (Major regulatory body). All medical devices will undergo conformity assessment procedures to ensure compliance with quality and safety standards before they are allowed to Indian markets. CLAA will adapt to regulatory Standards of Bureau of Indian Standards (BIS) and International Organization for Standards (ISO) specification for quality management For Class A devices - Manufacturers may perform their own conformity assessment procedures. For B, C, and D-CLAA in consultation with BIS publishes the list of notified bodies authorized to perform conformity assessment. Imported Medical devices that are already approved in the US/or and the EU Union or that have been deemed equivalent to CE mark and FDA approved device will be allowed on Indian market without undergoing separate conformity assessment procedure. Under the recently launched ‘Make in India’ project by Honourable Prime Minister of India, the need to raise global competitiveness of the Indian manufacturing sector and it is growth drivers for Indian Healthcare Industry. Few of them pertinent to Medical Technology Sector are:
• Fast-developing clinical capabilities with the country becoming a popular destination for Clinical Trials, contract research and manufacturing activities.
• Increase in India’s patient pool to over 10% in the next 10 years, mainly due to the rise in Population and rise in lifestyle diseases.
• Over USD 200 billion to be spent on medical infrastructure in the next decade.
• Rising levels of education to increase acceptability of medical technology
Recommendation to government
The government should take necessary steps to increase the growth of Indian market and stand amongst the top countries of the medical device market. There are few recommendations that we would like to give:
► Negotiate strongly to reduce or eliminate tariffs on medical devices
► Encourage greater collaboration between medical and technology universities.
► Increase the quality and enhance consistency of training received by medical and paramedical staff
► Increasing investments in smaller companies on how to comply with foreign regulatory requirements
► Authorities should set up global classification of IVD medical devices.
► Rules establish by authorities must be clear so that companies can easily classify their products.
► Post-market surveillance/vigilance should be made essential
► Provide export support opportunities similar to what foreign governments
► Accessories like kits should be classified separately so it’s easy for manufacturers.
Conclusion
The global medical device market is growing very fast from last decade and India had shown tremendous growth in this market and it is expected to grow in upcoming years. Indian regulators should take necessary steps to improve Indian medical device market and increase its growth globally. The private companies are to be more regulated and higher penalties should be decided so that companies follow correct regulations. Centralized procedures should be made in India so that rules are same for all. The government should work on accessibility and affordability of IVD’s.
Acknowledgements
The authors are thankful to Dr. RK Goyal, Vice Chancellor and Dr. DP Pathak, Registrar of Delhi Pharmaceutical Sciences and Research University, New Delhi, India for providing all the facilities and encouragement to carry out the work.
Conflict of Interest
The authors declare that there are no conflicts of Interest.
REFERENCES
- Indian pharmaceutical Industry, Executive Summary, Working group paper, planning commission, Available at: 2016 http://planningcommission.gov.in/aboutus/committee/wrkgrp12/wg_pharma2902.pdf
- Medical devices. Available at: 2016 http://www.who.int/medical_devices/02_improving_healthcare_it_systems_lisa_spellman.pdf
- The Indian medical device in India available at: 2016 www.skpgroup.com
- Indian medical devices report Espicom 2016.
- Global top 10 companies based on in vitro diagnostics revenue available at: http:/data.worldbank.org
- India Pharma Summit 2014-15 “Focus on critical verticals to foster access to pharmaceuticals, enhancing production and technology adoption in pharmaceutical enterprises and medical devices and diagnostics” Ramada plaza grove, Mumbai 2015.
- Nishith Desai Associates, The Indian Medical Device Industry, Regulatory, Legal and Tax Overview 2016.
- GHTF guidance document entitled “principles of conformity assessment for IVD medical devices” available at: http://www.imdrf.org/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n78-2012-conformity-assessment-medical-devices-121102.pdf
- GHTF (2008b) Principles of In-Vitro Diagnostic (IVD) Medical Devices Classification 2016.
- Regulatory guidance for medical device 2016. http://cdsco.nic.in/forms/list.aspx?lid=1674&Id=1
- Medical device diagnostic division of CDSCO. Available at: http://cdsco.nic.in/forms/list.aspx?lid=1580
- The Government of India, Ministry of Health and Family Welfare (Department of Health), number GSR 703(E), in the Gazette of India, Extraordinary, Part II, 2013.
- ITA Medical Devices Top Markets Report 2016; 1-9.
- Indian medical device outlook from: http://www.visiongain.com/Report/933/Indian-Medical-Device-Market-Outlook-2013-2023
- Ames Gross and Arthur Chyan, India’s Latest Medical Device Regulation Developments, Pacific Bridge Med 2011.
- Medical Devices & Diagnostics Equipment in India. Research and Markets 2016.
- Paddock, Richard. Medical Devices Regulatory Profile of India. International Trade Administration 2010.
- FMI wearable medical device market for the period 2016-2026.
- Medical device economic report available at: http://health.economictimes.indiatimes.com/news/industry/medical-devices-are-different-from-drugs-and-should-be-treated-differently-pavan-choudary/52335751
- Future medical device growth report: http://health.economictimes.indiatimes.com/health-files/future-growth-of-medical-device-market-in-india-as-compared-to-the-global-market/628