Controlled release matrix uncoated tablets of enalapril maleate using HPMC alone
- *Corresponding Author:
Date of Received: 16-02-2010
Date of Modified: 10-03-2010
Date of Accepted: 30-03-2010
Available Online: 15-05-2010
Abstract
Hydroxy propyl methyl cellulose (HPMC) is generally combined with hydrophobic polymers in fabricating oral controlled solid dosage forms. This study evaluated the utility of diverse grades of HPMC in developing a controlled release formulation for a hydrophilic drug, enalapril maleate. Controlled release uncoated tablets were prepared by direct compression technique. Two grades of HPMC (K100 and K4M) in different proportions were used to prepare the tablets, and were evaluated for physical properties, drug content, in vitro drug release and drug release kinetics as well. All the formulations demonstrated good physical integrity and the drug content were in the official limits. The formulation with HPMC K100 (25 mg/tablet) and K4M (15 mg/tablet) have been found to release the required amount of drug (2.97 mg/h) through out the study period (14 h). The calculated regression coefficients showed higher r2 value with Higuchi model and zero order kinetics. Given the excellent release profile, the study concluded that HPMC in different grades with low concen-tration alone can control the enalapril maleate release over a period of time (14 h).
Keywords
Enalapril maleate, Controlled release, Hydroxy propyl methyl cellulose, Matrix tablets.
Introduction
Matrix controlled release formulations are getting much attention in recent past due to higher clinical efficacy and patient compliance. Consequently, a range of controlled release dosage forms have been developed or are under development for the treatment of chronic conditions like hypertension. The ultimate aim in the design of an oral controlled release dosage form includes maintaining relatively constant therapeutic blood levels of the drug for a desired period, reduce dosing frequency, improve patient compliance and decrease incidences of adverse drug reactions [1].
Pharmaceutical literature suggests that hydrophilic matrix system is widely used for the modification of drug release in oral therapy because of its simplicity in manufacture [2,3]. In such a system, the drug release is controlled by a combination of several physical processes which include, but are not limited to, diffusion, polymer swelling, erosion and dissolution. A successful hydrophilic matrix system should possess a polymer that will wet, hydrate and swell to form a gelatinous layer and avoid disintegration of the tablet. To achieve this, different cellulose derivatives or their combinations have been extensively used in the preparation of matrix tablets. Hydroxy propyl methyl cellulose (HPMC) is the most widely studied hydrophilic swellable matrix forming material for the preparation of modified drug release products. Its popularity can be attributed to the polymer’s non-toxic nature, small influence of processing variables on drug release, ease of compression, and its capability to accommodate high levels of drug loading [1,4].
Hypertension being a chronic disorder requires a steady state concentration through out the treatment. Enalapril maleate (EM), an ACE inhibitor is commonly administered orally during the treatment for hypertension. However, due to its low oral bioavailability (41%), short biological half life and narrow therapeutic concentration, the dosing management of the drug has become convoluted [5]. The objective of the present study was to formulate and evaluate a controlled release matrix uncoated tablets for EM using varying viscosity grades of HPMC (K100 and K4M).
Materials and Methods
Materials
EM (Ranbaxy, Gurgaon, India), HPMC K100 and HPMC K4M (Colorcon, Goa, India) were received as gratis samples. All other additives like acetonitrile and water (HPLC grade), spray dried lactose, poly vinyl pyrrolidone K30, magnesium stearate were procured commercially.
Preparation of matrix tablet of Enalapril maleate
The detailed compositions of EM matrix tablet formulations are given in Table 1. Matrix tablets were prepared by direct compression method. The respective powders (drug, polymer, and additives) were passed through a sieve no. 60 (250 μm) and blended with a turbula mixer. All the batches were compressed on 10 station tablet machine (Cadmach, Ahmedabad, India) with 9 mm flat round punches. Three batches were prepared for each formulation.
| Ingredients (mg) | Formulation batch | |||
|---|---|---|---|---|
| E1 | E2 | E3 | E4 | |
| Enalapril maleate | 40.00 | 40.00 | 40.00 | 40.00 |
| HPMC K100 | 25.00 | 05.00 | 25.00 | 15.00 |
| HPMC K4M | 05.00 | 25.00 | 15.00 | 25.00 |
| Spray dried lactose | 218.00 | 218.00 | 208.00 | 208.00 |
| Poly vinyl pyrrolidone K30 | 09.00 | 09.00 | 09.00 | 09.00 |
| Magnesium stearate | 03.00 | 03.00 | 03.00 | 03.00 |
Table 1: Composition (in mg/tablet) of 40 mg enalapril maleate matrix tablets
Characterization of tablets
The weight variation of the tablets were performed by randomly selecting twenty tablets and weighed individually and together in a single pan balance (Shimadzu, AX200, Japan). Friability was tested by Roche friabilator (Electro Lab, EF-2, Mumbai, India). Preweighed tablets were allowed for 100 revolutions in four minutes and were dusted. The percentage loss was calculated by reweighing the tablets. Hardness was tested by commonly used Monsanto type tablet hardness tester (IEC, Mumbai, India) by placing a tablet between the anvils and the crushing strength, which causes the tablet to break, was recorded. The thickness of the tablets was measured with a vernier caliper [6].
Drug content studies
The drug content in tablets were determined by randomly choosing five tablets of each formulation and thinly minced in a mortar separately. A quantity equivalent of 10 mg of EM was weighed, dissolved in mobile phase diluted suitably. The amount of drug was determined by injecting 20 μl of the sample in a high performance liquid chromatography system (HPLC; Shimadzu, LC-10ATVP, Kyoto, Japan). The HPLC system consists of a Phenomenex C18 analytical column (4.6 × 250 mm, Luna, 5.0 μm). The column was maintained at ambient temperature. The compounds were eluted at a flow rate of 1 ml/min using a mobile phase of acetonitrile-water (20:80) [7]. The column effluent was monitored at 215 nm and the retention time was found to be 2.2 min. The method was validated by determination of linearity, precision, and accuracy. The range for the calibration curve was 4 − 800 ng/mL (R2 = 0.98). The coefficient of variation and the relative error ranged 0.61− 5.93% and -0.62 − -8.52%, respectively.
In vitro drug dissolution studies
Drug release was evaluated in vitro using USP XIII Type II dissolution test apparatus (Electro Lab, TDT-08L, Mumbai). The dissolution for all the formulations was carried out in 750 ml 0.1 N HCl for first 2 h and then made to 1000 ml with phosphate buffer to a pH of 6.8 and for a period of 12 h [8]. The temperature was maintained at 37 ± 0.5°C and a constant paddle rotation speed of 50 rpm. Sink condition was maintained throughout the studies. Samples (5 ml) were withdrawn at regular intervals and filtered through membrane filter (pore size 0.22 μm). The sample solutions were further diluted and analyzed for EM by HPLC method as previously described in drug content studies.
Kinetics of drug release
The in vitro release data of the controlled release matrix tablets were evaluated kinetically by zero order kinetics, first order kinetics, Higuchi models and Hixson Crowell and the ideal kinetic models were estimated for drug release using the following equations [9].
Zero order kinetics: Ft = K0t where Ft represents the fraction of drug released in time t and K0 the apparent release rate constant or zero order release constant.
First order kinetics: ln (1−F) = −K1t where F represents the fraction of drug released in time t and K1 is the first order release constant.
Higuchi model: F = K2t1/2 where F represents the fraction of drug released in time t and K2 is the Higuchi dissolution constant.
Hixson–Crowell model: W01/3 - Wt1/3= Kst where W0 is the initial amount of drug in the matrix tablets, Wt is the remaining amount of drug in the matrix tablets at time t and Ks is a constant incorporating the surface volume relation. Dividing the above equation by W01/3 and simplifying:
(1−F)1/3 = 1−K3t
where F = 1 − (Wt/W0) and F represents the drug dissolved fraction at time t and K3 is the release constant.
Statistical analysis
All the data obtained for dissolution and drug content were evaluated statistically. The data were tested by one-way analysis of variance (ANOVA) and t-test using Graphpad prism 5, graphpad software, Inc., CA, USA, to test the effects of various treatments. P value less than 0.05 was considered statistically significant. The data points provided in the graph is an average of six trials. The error bars represents the standard deviation.
Results and Discussion
HPMC is the most widely used polymer for oral controlled delivery due to its pH independent drug release [10]. Its mechanism is well known as a swelling controlled release system and the release of hydrophilic drugs from this polymer is by diffusion process [11]. Generally it is used in combination with other polymers. In this study we have used two grades of HPMC (K100 and K4M) with varying quantities. Spray-dried lactose, polyvinyl pyrrolidone K30, and magnesium stearate are used as diluent, binder, and lubricant, respectively. Four formulations of EM controlled-release matrix tablets were prepared by direct compression technique. The total weight of the formulation was kept constant (300 mg), while the amount of HPMC and spray dried lactose were varied. As an ideal controlled release formulation should contain polymers the least possible amount and release the content in a controlled manner over a reasonable time, therefore a minimum quantity of polymers were used (Table 1).
The physical properties of compressed controlled release tablets, such as weight variation, friability, hardness, and thickness, were determined by using standard protocols. The results obtained were recorded in Table 2. It can be seen that the weight variation obtained for all the formulations were well in the range of official limit. The friability studies revealed that there was no signifi- cant difference among the formulations and have good physical integrity (Table 2). The hardness of the tablets also did not vary significantly (4.02– 4.95 kg/cm2). Similarly, no significant differences in thickness values were observed between different formulations. The drug content study proved that all the formulations had a better uniformity, and the assay values were statistically insignificant. The amount of drug in the formulations did not vary by more than 2% and were in the range of 98.27 to 101.48%.
| Ingredients | Formulation batch | |||
|---|---|---|---|---|
| E1 | E2 | E3 | E4 | |
| Weight (mg) | 294 ± 3.35 | 295 ± 5.18 | 296 ± 4.25 | 292 ± 3.37 |
| Friability (%) | 0.83 ± 0.15 | 0.82 ± 0.21 | 0.81 ± 0.17 | 0.85 ± 0.28 |
| Hardness (kg/cm2) | 4.76 ± 1.14 | 4.02 ± 0.88 | 4.95 ± 1.02 | 4.25 ± 0.75 |
| Thickness (cm) | 3.20 ± 0.44 | 3.40 ± 0.35 | 3.12 ± 0.22 | 3.33 ± 0.23 |
*Values are the mean ± SD (n = 3).
Table 2: Physical properties* of formulated enalapril maleate matrix tablets
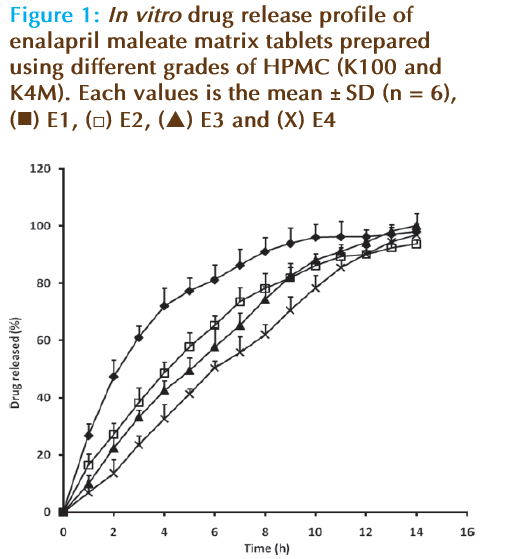
According to the theoretical release profile worked out by using the pharmacokinetic data, a constant supply of 2.97 mg/h (7.42% per hour for 40 mg dosage) is required to be released (desired release). Fig. 1 represents the in vitro drug release profiles of all the four prepared formulations of controlled release matrix tablets. It is evident from the figure that the drug release (through out the study period) by the prepared formulations were more or less steady. In formulation E1, where greater amount of HPMC K100 (25 mg/tablet) was used when compared to K4M (5 mg/tablet), the release rate was higher from the initial period with greater amount being released (>90%) in 6 h. Hence in the next batch (E2), the quantity of polymer was interchanged, i.e., HPMC K100 (5 mg/tablet) and K4M (25 mg/tablet). Fig. 1 also indicated that the increase in the K4M quantity could not yield the desired release profile, but the extent of drug release was reduced when compared to formulation E1. This data suggests that the concentration of polymers (HPMC K 100 and K4M) used were inadequate to control the release of EM.
Hence, another two batches of matrix tablets were prepared with greater amount of polymers [HPMC K100 (25 mg/tablet) and K4M (15 mg/ tablet) in batch E3 and HPMC K100 (15 mg/tablet) and K4M (25 mg/tablet) in batch E4]. Interestingly, the required amount of drug release was observed in formulation E3 (Figure 1), through out the study period (14 h). The basic difference between E1 and E3 formulation is regarding the amount of HPMC K4M, greater amount (3 fold) with the latter. Thus the slow release of EM from E3 could be essentially due to the HPMC K4M (polymer) characteristics (high concentration, high viscosity, high molecular weight, greater binding capacity and superior cross linking), in the current experimental condition. Further, the release was significantly controlled in formulation E4, but required drug release was not achieved in the initial stage of the study. The above data suggests that the increase in high molecular weight HPMC polymer concentration could reduce the drug release significantly. Separately, the percentage of drug released from formulations E1, E2, E3 and E4 at 6 h were found to be 81, 65, 57 and 50%, respectively (Figure 1).
Further, the prepared formulations were analyzed for the drug release kinetically. The calculated regression coefficients showed higher r2 value with Higuchi models and the values were 0.953, 0.950, 0.997 and 0.995 for formulations E1, E2, E3 and E4, respectively. When analyzed for zero order, the r2 values were found to be 0.910, 0.951, 0.984 and 0.987 for E1, E2, E3 and E4 formulations, respectively. However, the regression values were found to be low with first order kinetics and Hixson Crowell models. Thus, both zero-order and Higuchi models could be applicable, although the drug release from the formulation E3 showed acceptable linearity (higher r2 value for the whole release process) and fit with the Higuchi model equation of diffusion from the matrix. In nutshell, it can be concluded that using HPMC in different grades with low concentration alone can control; the EM release for a period of 14 h and the dissolution follows Higuchi model.
References
- Khairuzzaman A, Ahmed SU, Savva M, Patel NK. Zero-order release of aspirin, theophylline and atenolol in water from novel methylcellulose glutarate matrix tablets. Int J Pharm. 2006; 318: 15-21.
- Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled release dosage forms. Int J Pharm Tech Prod Manuf. 1984; 5: 1–9.
- Deshapande AA, Rhodes CT, Shah NH. Controlled release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm. 1996; 22: 531–539.
- Gil EC, Colarte AI, Bataille B, Pedraz JL, Rodriguez F, Heinamaki J. Development and optimization of a novel sustained release dextran tablet formulation for propranolol hydrochloride. Int J Pharm. 2006; 317: 32-39.
- Brunton LL, Lazo JS, Parker KL, eds. Goodman and Gilman’s The Pharmacological basis of therapeutics. New York, McGraw-Hill, 2006.
- Kuksal A, Tiwary AK, Jain NK, Jain S. Formulation and in vitro, in vivo evaluation of extended release matrix tablets of Zidovudine: Influence of combination of hydrophilic and hydrophobic matrix formers. AAPS Pharm Sci Tech. 2006; 7: E1-E9.
- Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behavior, mechanisms and optimal performance. Pharm Sci Technol Today 2000; 3: 198-204.
- Keith M. Compression and consolidation of the powdered solids. In: Leon Lachman, Liberman HA, Kanig JL. (eds). Theory and practice of Industrial Pharmacy. ed 3, Mumbai, India: Varghese publishing house, 1991, 66-72.
- Walily AM, Belal SF, Heaba EA, Kersh AE, Simultaneous determination of enalapril maleate and hydrochlorothiazide by first-derivative ultraviolet spectrophotometry and high-performance liquid chromatography. J Pharm Biomed Anal. 1995; 13: 851-856.
- US Pharmacopoeia XXIV, Rockville, MD, 2000.
- Hayashi T, Kanbe H, Okada M, Suzuki M, Ikeda Y, Onuki Y, Kaneko T, Sonobe T. Formulation study and drug release mechanism of a new theophyline ustained-release preparation. Int J Pharm. 2005; 304: 91-101.