Comparative evaluation of subgingivally delivered chlorhexidine varnish and chlorhexidine gel in reducing microbial count after mechanical periodontal therapy
- *Corresponding Author:
- Dr. Sathish Manthena
H. No. 23-39-50/A, Jagarlamudi Street, Lakshmi Nagar, Satyanarayanapuram, Vijayawada - 520 011, Andhra Pradesh, India.
E-mail: sathishmanthena@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Context: Antimicrobial efficacy of subgingival chlorhexidine (CHX) application using two different vehicles of delivery. Aims: The aim was to evaluate the efficacy of CHX varnish and gel as an adjunct to scaling and root planing (SRP) in reducing microbial count within moderate to deep periodontal pockets. Settings and Design: Experimental parallel mouth study. Subjects and Methods: A total of 30 subjects between the age groups 25 and 55 years having moderate to severe periodontitis, with pocket depth ≥ 5 mm were selected for the study. The selected patients were randomized into three groups of 10 each. Subjects in Group 1 received SRP followed by subgingival application of CHX varnish, subjects in Group 2 received SRP followed by subgingival application of CHX gel, subjects in Group 3 received SRP alone. Subgingival plaque samples were collected to estimate mean motile and nonmotile microbial counts using dark field microscopy at baseline, 1 week, 1 month, and 3 months. Results: After 3 months, there was statistically significant reduction in nonmotile microbial count in all the three groups. Motile microbial count was significantly reduced in all the three groups till 1 month from baseline. Only subjects in Group 1 who received subgingival CHXvarnish after SRP showed a significant reduction in motile microbial count till 3 months from baseline. Conclusions: Subgingival application of highly concentrated CHX varnish following SRP is beneficial in reducing microbial count in moderate to deep periodontal pockets.
Keywords
Chlorhexidine, gel, periodontitis, scaling and root planing, varnish
Introduction
In recent years, it has been well-documented that microorganisms play an important role in the etiology of periodontal diseases, and specific microorganisms are responsible for specific disease processes. [1] Hence, successful periodontal therapy is dependent on antiinfective procedures, aimed at eliminating pathogenic organisms found in dental plaque associated with the tooth surface and within other niches in the oral cavity. Currently, the most common therapy for periodontal inflammatory diseases consists of repeated professional supra and subgingival plaque removal through scaling and root planing (SRP). [2] However, in deep pockets and furcations, SRP was found to be of limited efficacy. Therefore, treatment strategies using antimicrobials in conjunction with SRP have been evolved. [3]
As an antiseptic, chlorhexidine (CHX) has been used effectively for over 30 years in the treatment of periodontal disease. It shows a broad spectrum of topical antimicrobial activity, safety, effectiveness, substantivity, and lack of toxicity. [4] A number of vehicles delivering CHX has been developed. Vehicles that can be used to administer CHX subgingivally are mouth rinses, gels, varnishes, and gelatin chip (periochip).[5]
Aim of this present study was to compare the efficacy of subgingival application of CHX varnish and CHX gel as an adjunct to full mouth SRP in reducing the microbial count of moderate to deep periodontal pockets.
Subjects and Methods
This study was conducted in the Department of Periodontics, A.B. Shetty Memorial Institute of Dental Sciences, Derlakatte, Mangalore. The study consists of 30 subjects, 11 males and 19 females between the age groups 25 and 55 years having moderate to severe periodontitis (according to AAP classification 1999).
Criteria for patient selection
Subjects with moderate to severe chronic periodontitis (AAP classification 1999) with at least three nonadjacent interproximal sites having a probing pocket depth ≥ 5 mm, with no contraindication to periodontal therapy were included in the study. Patients were required to not have received any periodontal and antibiotic therapy in the last 6 months and were to be cooperative and committed to maintain oral hygiene. Patients who were smokers, tobacco, and alcohol abusers, with any systemic illness, pregnant and lactating females, using an antimicrobial mouthwash in the past 2 months, and those requiring periodontal surgery were excluded from the study.
Study design
It was an examiner blinded (treatment and microbial analysis were done by two examiners blindedly) parallel design clinical trial. The subjects qualified for the study were selected. The nature and design of the clinical trial were explained to the patients, and consent was obtained in writing for their participation. Then subjects were assigned randomly (in order to eliminate personal bias in selection of the sample, simple random sampling, i.e. lottery method was used) into one of the three groups of 10 subjects each. In subjects belonging to Group 1, SRP was followed by full mouth subgingival application of 20% concentrated CHX varnish.
In subjects belonging to Group 2, SRP was followed by full mouth subgingival application of 1.5% concentrated CHX gel and in subjects belonging to Group 3, only SRP was done. The patients were then instructed not to use any other chemical plaque control methods other than routine brushing and rinsing with water.
The materials used in the study were the BioC (CHX varnish); it was a local drug delivery system (Biodent BV, Netherlands). BioC is a supersaturated solution of 20% CHX diacetate in alcohol. The solution is stabilized by sandarac a resin. The chlosite gel (CHX gel) is a local drug delivery system (GHIMAS, Italy). It contains CHX in xanthan gel. The gel is a unique combination of two formulations, CHX digluconate 0.5% and CHX dihydrochloride 1%.
A syringe with a blunt needle was used for application of CHX varnish and gel. The varnish and gel were released slowly while the needle was moved in a coronal direction from the bottom of the pocket. About 15 min following their application the varnish and gel were gently removed using a gracey curette.
Microbiologic procedure
After isolating the selected sites, subgingival plaque samples were collected from each patient using a sterile curette from the periodontal pocket and mixed with a prereduced anaerobically sterilized ringers solution. Samples were dispersed by vortexing at maximal setting for 60 s.
Microscopic examination
Within 1 hour of sampling, a drop of the dispersed plaque sample was mounted on a glass slide and examined under the phase contrast microscope at a magnification of ×400 for the presence and distribution of motile and nonmotile microbial count. For each plaque sample the microbial counts were made for two separate fields and subsequently their mean was calculated at baseline before starting the treatment, 1 week, 1 month, and at the end of 3 months.
Statistical analysis was done using paired t-test for intragroup and one way ANOVA test, Tukey HSD test. Intragroup comparison of posttreatment changes in microbial count was analyzed using paired t-test, and intergroup comparison of posttreatment changes in microbial count was analyzed using one-way ANOVA test and Tukey HSD test.
Paired t-test was calculated by
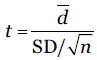
d is the mean of difference, SD/√n is the standard error of the difference.
Tukey HSD test
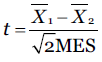
t is the Tukey HSD, X1 the mean of periodontal disease group, X2 the mean of the control group, and MES is the mean error sum of square which we obtain from ANOVA.
Results
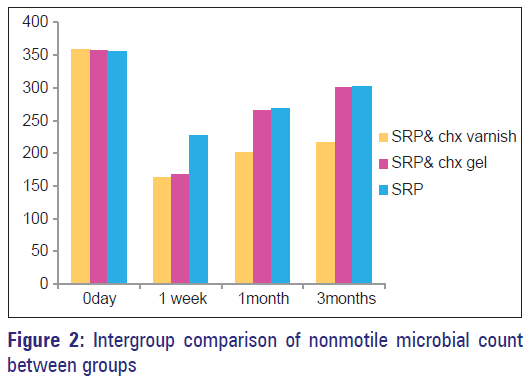
Mean percentage of nonmotile microbial count in Group 1, Group 2, and Group 3 from baseline to 3 months were outlined in [Table 1]. On the comparison of nonmotile microbial counts within the groups at different intervals, all the three groups showed a significant reduction from baseline to 3 months [Table 1].
| Group | Observation period | Mean±SD | Std. Error | Comparison | Mean difference | t | P |
|---|---|---|---|---|---|---|---|
| 1 (Chx varnish+SRP) | Baseline (BL) | 359.01±45.53 | 14.39 | ||||
| 1 week (1W) | 163.24±33.98 | 10.74 | BL Vs 1W* | 195.76 | 12.29 | 0.000 | |
| 1 month (1M) | 202.43±43.53 | 13.76 | BL Vs 1M* | 156.57 | 8.49 | 0.000 | |
| 3months (3M) | 216.60±33.44 | 10.57 | BL Vs 3M* | 142.40 | 8.97 | 0.000 | |
| 2 (Chx gel+SRP) | Baseline (BL) | 358.74±51.88 | 16.40 | ||||
| 1 week (1W) | 167.40±61.94 | 19.58 | BL Vs 1W* | 191.33 | 9.85 | 0.000 | |
| 1 month (1M) | 267.35±60.46 | 19.12 | BL Vs 1M* | 91.38 | 4.41 | 0.002 | |
| 3months (3M) | 301.54±41.25 | 13.04 | BL Vs 3M* | 57.19 | 2.57 | 0.030 | |
| 3 (SRP) | Baseline (BL) | 356.52±46.54 | 14.71 | ||||
| 1 week (1W) | 228.51±34.34 | 10.85 | BL Vs 1W* | 128.01 | 6.51 | 0.000 | |
| 1 month (1M) | 269.04±30.37 | 9.60 | BL Vs 1M* | 87.47 | 4.48 | 0.002 | |
| 3months (3M) | 303.39±26.59 | 8.41 | BL Vs 3M* | 53.12 | 2.98 | 0.015 |
P value significant at ≤0.05. *Significant difference. SD: Standard deviation
Table 1: Comparison of mean nonmotile microbial count at different observation periods in different groups
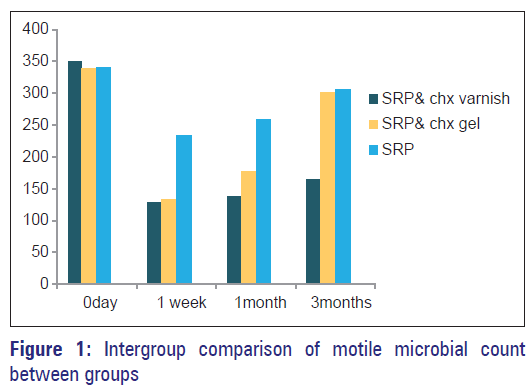
Mean percentage of motile microbial count in the three groups at different observation periods were outlined in [Table 2]. On the comparison of motile microbial counts within the groups at different intervals, only subjects in Group 1 (CHX varnish + SRP) showed a significant reduction even at 3 months from baseline. Group 2 (CHX varnish + SRP) and Group 3 (SRP) showed a significant reduction only till 1 month from baseline.
| Group | Observation period | Mean±SD | Std. Error | Comparison | Mean difference | t | P |
|---|---|---|---|---|---|---|---|
| 1 (Chx varnish+SRP) | Baseline (BL) | 349.89±45.17 | 14.28 | ||||
| 1 week (1W) | 129.61±26.09 | 8.25 | BL Vs 1W* | 220.27 | 16.71 | 0.000 | |
| 1 month (1M) | 137.48±25.60 | 8.09 | BL Vs 1M* | 212.41 | 15.08 | 0.000 | |
| 3months (3M) | 164.03±30.55 | 9.66 | BL Vs 3M* | 185.85 | 11.33 | 0.000 | |
| 2 (Chx gel+SRP) | Baseline (BL) | 340.30±48.66 | 16.74 | ||||
| 1 week (1W) | 133.28±23.66 | 8.04 | BL Vs 1W* | 207.02 | 11.40 | 0.000 | |
| 1 month (1M) | 178.24±21.30 | 7.43 | BL Vs 1M* | 162.06 | 9.05 | 0.000 | |
| 3 months (3M) | 302.06±36.79 | 12.97 | BL Vs 3M | 38.24 | 1.58 | 0.151 | |
| 3 (SRP) | Baseline (BL) | 341.80±40.03 | 12.66 | ||||
| 1 week (1W) | 235.06±25.43 | 8.04 | BL Vs 1W* | 106.73 | 6.36 | 0.000 | |
| 1 month (1M) | 260.79±25.09 | 7.93 | BL Vs 1M* | 81.00 | 5.28 | 0.001 | |
| 3 months (3M) | 307.63±21.68 | 6.85 | BL Vs 3M | 34.16 | 2.24 | 0.051 |
*P value significant at ≤0.05. SD: Standard deviation, SRP: Scaling and root planing
Table 2: Comparison of mean motile microbial count at different observation periods in different groups
Multiple comparison (intergroup) of motile microbial count between three groups at different intervals were outlined [Table 3, Figure 1]. At baseline, there was no significant difference between the three groups. Group 1 (CHX varnish + SRP) when compared to Group 2 (CHX gel + SRP) and Group 3 (SRP), showed a statistically significant reduction in motile microbial count at 1 month, 3 months and 1 week, 1 month, and 3 months respectively. On comparing Group 2 (CHX gel + SRP) with Group 3 (only SRP), significant difference in motile microbial count was seen only at 1 week and 1 month.
| Dependent variable | (I) Groups | (J) Groups | Mean difference | P |
|---|---|---|---|---|
| Base line | 1 | 2 | 9.5860 | 0.882 |
| 3 | 8.0930 | 0.914 | ||
| 2 | 3 | −1.4930 | 0.997 | |
| 1 week | 1 | 2 | −3.6650 | 0.943 |
| 3 | −105.4460* | 0.000 | ||
| 2 | 3 | −101.7810* | 0.000 | |
| 1 month | 1 | 2 | −40.7600* | 0.002 |
| 3 | −123.3150* | 0.000 | ||
| 2 | 3 | −82.5550* | 0.000 | |
| 3 months | 1 | 2 | −138.0240* | 0.000 |
| 3 | −143.5980* | 0.000 | ||
| 2 | 3 | −5.5740 | 0.911 |
*P value significant at ≤ 0.05
Table 3: Intergroup comparison of motile microbial count
Multiple comparison (intergroup) of nonmotile microbial count between three groups at different intervals were outlined [Table 4, Figure 2]. There was no statistically significant difference between all the three groups at baseline. On comparing nonmotile microbial count, between Group 1 (CHX varnish + SRP), Group 2 (CHX gel + SRP) and Group 3 (SRP), a significant reduction in nonmotile microbial count was seen at 1 month, 3 months, and at 1 week, 1 month, and 3 months, respectively. Group 2 (CHX gel + SRP) when compared to Group 3 (SRP) significant difference in nonmotile microbial count was seen only at 1 week.
| Dependent variable | (I) Groups | (J) Groups | Mean difference | P |
|---|---|---|---|---|
| Base line | 1 | 2 | 0.2700 | 1.000 |
| 3 | 2.4900 | 0.993 | ||
| 2 | 3 | 2.2200 | 0.994 | |
| 1 week | 1 | 2 | −4.1610 | 0.977 |
| 3 | −65.2650* | 0.009 | ||
| 2 | 3 | −61.1040* | 0.015 | |
| 1 month | 1 | 2 | −64.9250* | 0.011 |
| 3 | −66.6120* | 0.009 | ||
| 2 | 3 | −1.6870 | 0.996 | |
| 3 months | 1 | 2 | −84.9370* | 0.000 |
| 3 | −86.7850* | 0.000 | ||
| 2 | 3 | −1.8480 | 0.992 |
*P value significant at ≤ 0.05
Table 4: Intergroup comparison of nonmotile microbial count
Discussion
The results obtained in the present randomized trial showed that the adjunctive subgingival administration of CHX varnish significantly reduced microbial counts even after 3 months. In the present study to evaluate the duration of any beneficial effects of CHX varnish and gel as an adjunct to SRP, no further subgingival treatment was provided after baseline therapy. The 3 months interval was chosen because in an in vitro study CHX varnish concentration of about 50 μg/ml was maintained constantly over a 3-month period, which is higher than the minimal inhibitory concentration of CHX. [6] Furthermore, 3 months corresponds to the typical recall interval for patients after periodontal treatment. [7]
The method of microbial analysis using Darkfield microscopy employed in this study reveals the shapes of bacteria and their motility, but does not permit any identification of bacterial classification or species. The method is, therefore, limited in its informative potential.
In the present study, only subjects who received subgingival application of CHX varnish after SRP (Group 1) showed statistically significant reduction in mean motile count even at 3 months when compared to baseline. These findings are in accordance with the studies conducted by Cosyn et al., [5] Mızrak et al. [8] and Schaeken et al. [9] In a study by Schaeken et al. 12 subjects treated with a 50% CHX varnish showed a significant suppression of S. mutans in the interdental plaque until the 4th week. [9]
The results of the present study showed that a treatment strategy, supplementing mechanical debridement by subgingival CHX varnish application provides significantly greater reduction in motile and nonmotile microbial count compared to those obtained by subgingival application of CHX gel after SRP and SRP alone. These findings are in accordance with the results obtained in the studies conducted by Cosyn [5] and Shapira et al. [10]
But these findings are in contrast to the study by Vivien B Dudic. [11] In his study, CHX-thymol varnish treatment as an adjunct to mechanical periodontal therapy, had little effect on the periodontal condition and the microbial flora in subjects with an existing good oral hygiene. [11]
Subjects who received subgingival application of CHX gel after SRP when compared to subjects who received only SRP showed a significant reduction in microbial count only till 1 month. After 3 months, there was no significant difference between the two groups. These findings are in accordance with studies conducted by Oosterwaal et al. [12]
Even though it seems logical that CHX varnish is more effective than CHX gel due to its high CHX concentration (20% of for varnish vs. 1.5% for the gel), one should keep in mind that this is a small-scale trial presenting preliminary data. Lack of a long-term antibacterial effect of the gel may be explained by the short exposure time and a single application procedure. If the gel and varnish were left in place for as long as it can adhere, the effect might be different.
While these short-term results suggest that antimicrobial agents for the treatment of chronic periodontitis are efficacious, evidence is needed for long-term benefits to determine the full potential of these treatment modalities. The limitations of the present study were lower sample size, duration of the study, and the method of microbial analysis. This method of microscopic examination does not require fixation or gram staining and are, therefore, quick and simple to perform. But this method provides limited conclusion about pathogenecity of microorganisms. To optimize the above clinical procedures, future research should focus on pharmacokinetics and pharmacodynamics to clarify the mode of action of a supersaturated CHX varnish within subgingival area.
Acknowledgment
The authors would like to thank Biodent, Netherlands and GHIMAS Italy for supplying the varnish and gel.
References
- Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol 1996;1:821-78.
- Badersten A, Nilve´us R, Egelberg J. Effect of non-surgical periodontal therapy in moderately advanced periodontitis. J Clin Periodontol 1981;8:57-72.
- Rateitschak-Plüss EM, Schwarz JP, Guggenheim R, Düggelin M, Rateitschak KH. Non-surgical periodontal treatment: Where are the limits? An SEM study. J Clin Periodontol 1992;19:240-4.
- Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res 1970;5:79-83.
- Cosyn J, Sabzevar MM. Subgingival chlorhexidine varnish administration as an adjunct to same-day full-mouth root planing. II. Microbiological observations. J Periodontol 2007;78:438-45.
- Huizinga ED, Ruben JL, Arends J. Chlorhexidine and thymol release from a varnish system. J Biol Buccale 1991;19:343-8.
- Petersson LG, Edwardsson S, Arends J. Antimicrobial effect of a dental varnish, in vitro. Swed Dent J 1992;16:183-9.
- Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol 2006;77:437-43.
- Schaeken MJ, van der Hoeven JS, Hendriks JC. Effects of varnishes containing chlorhexidine on the human dental plaque flora. J Dent Res 1989;68:1786-9.
- Shapira J, Sgan-Cohen HD, Stabholz A, Sela MN, Schurr D, Goultschin J. Clinical and microbiological effects of chlorhexidine and arginine sustained-release varnishes in the mentally retarded. Spec Care Dentist 1994;14:158-63.
- Dudic VB, Lang NP, Mombelli A. Microbiological and clinical effects of an antiseptic dental varnish after mechanical periodontal therapy. J Clin Periodontol 1999;26:341-6.
- Oosterwaal PJ, Mikx FH, vant Hof MA, Renggli HH. Comparison of the antimicrobial effect of the application of chlorhexidine gel, amine fluoride gel and stannous fluoride gel in debrided periodontal pockets. J Clin Periodontol 1991;18:245-51.