Are the optimum pharmaceutical preparations used for the second-line eradication therapy for helicobacter pylori infection in japan? - A discussion from a simulation for the amount of antibiotics in stomach based on the data of dissolution studies
- *Corresponding Author:
- Tadakazu Tokumura
Department of Pharmaceutical Sciences, School of Pharmacy, International University of Health and Welfare, 2600-1 Kitakanemaru, Ohtawara, Tochigi 324-8501, Japan
E-mail: tokumura2003@yahoo.co.jp
Date of Received : 09-03-2010
Date of Modified : 18-07-2010
Date of Accepted : 10-10-2010
Available Online : 15-11-2010
Abstract
The purpose of this study was to evaluate whether the optimum preparations are used for the second-line eradication therapy for Helicobacter pylori (H. pylori) infection in Japan. In the therapy, commercial tablets, which have 250 mg of amoxicillin (AMX) and 250 mg of metronidazole (MTZ), are used as antibiotics. The evaluation was performed by dissolution tests and simulations for intragastric drug concentrations based on the dissolution data. The dissolution tests were performed using the paddle method of Japanese pharmacopoeia XV. The data obtained were used for the simulation of intragastric drug concentrations. In dissolution studies, the 100% dissolution time (T100%) of AMX from the tablet which was about 20 min was faster than that from AMX alone (about 6 h). T100% of MTZ from the tablet which was about 3 h was later than that from MTZ alone (about 30 min). In simulated intragastric concentrations, the time above minimum inhibitory concentration (MIC) of the AMX tablet was 6.6 h which was shorter than that of AMX (11.4 h). The Cmax of the MTZ tablet was 0.095 mg/ml which was lower than that of MTZ (0.190 mg/ml). However, AMX is a timedependent antibiotic, longer duration above MIC is desirable. On the other hand, MTZ is a concentration-dependent antibiotic, higher Cmax is desirable. In conclusion, the commercial products are found to be not the best, and preparing an AMX pharmaceutical preparation with more sustained release characteristic and a MTZ preparation with more rapid release characteristic are considered to be very advantageous
Keywords
Amoxicillin; Metronidazole; Helicobacter pylori; Dissolution test; Simulation
Abbreviations
AMX – Amoxicillin
CAM – Clarithromycin
FLG – Flagyl
H. pylori – Helicobacter pylori
JP – Japanese pharmacopoeia
MTZ – Metronidazole
MIC – Minimum inhibitory concentration
PST – Pasetocin Tablets 250
PPI – Proton pump inhibitor
TAM – Time above MIC
Introduction
Gastric Helicobacter pylori (H. pylori) infection is associated with chronic (type B) gastritis and peptic ulcer [1-3]. H. pylori are often observed to adhere to the antral epithelium of the human stomach and gastric metaplasia in the duodenum. Gastric and duodenal ulcers are believed to develop as the result of damage to the gastric mucosa by cytotoxic substances (ammonia, cytotoxin, etc.) produced by H. pylori [4]. In developed countries, at least 20% of people younger than 20 years old and 50% of those older than 60 years old are infected [5]. In developing countries, the rate of infection is much higher, with a prevalence of 85% in northern Nigeria and 71-79% in other African countries [6]. Even if gastric and duodenal ulcers with H. pylori infection are treated, it will most likely cause the recurrence of the ulcers [7-9]. Therefore, the eradication of H. pylori is considered to be very important.
The primary eradication therapy for H. pylori in Japan which is a triple therapy using amoxicillin (AMX), clarithromycin (CAM) and proton pump inhibitor (PPI) was started in 2000 with the therapy approved for health insurance coverage (10). However, because of the increase of incomplete eradication, the second-line eradication therapy was approved in 2007 (10). In the eradication therapy, metronidazole (MTZ) is used in the place of CAM. The H. pylori strains with CAM resistance signifi cantly increase more and more, so the importance of the second-line eradication therapy also increases [10].
On the other hand, the pharmaceutical preparations used in the eradication therapy in Japan were not developed for the eradication therapy. For example, Pasetocin Tablets 250 (PST) for AMX and Flagyl (FLG) for MTZ were developed in 1981 and 1963, respectively, for various bacterial infections. From the point of view of the design of pharmaceutical preparations, there is a question whether the used preparations are the optimum or not, because these preparations were designed to exert the effect through the blood circulation after absorbed from the GI tract. For H. pylori eradication therapy, a direct attack in stomach is expected [11].
Therefore, we selected the commercial pharmaceutical preparations used in second-line therapy due to the increasing importance, and performed the simulation studies for the intragastric concentrations of the antibiotics, AMX and MTZ from the results of dissolution test. And we tried to evaluate whether they are optimal preparations or not.
Materials and Methods
Materials
Amoxicillin (AMX) and metronidazole (MTZ) were purchased from Sigma Chemical Company (St. Louis, MO, USA) and Wako Pure Chemical Industries, Ltd. (Osaka, Japan), respectively. Pasetocin Tablets 250 (PST) and Flagyl (FLG) which were from Sigma Pharmaceuticals Pty. (Croydon, Australia) and Shionogi Pharmaceutical Co. (Osaka, Japan) were used as the pharmaceutical preparations for AMX and MTZ. Other chemicals were of special reagent grade or HPLC grade.
Dissolution test
Dissolution characteristics of AMX and MTZ, and those tablets were evaluated by the paddle method of Japanese pharmacopoeia (JP) XV at 50 rpm of paddle speed using JP XV apparatus. The media used were 900 ml of the first and the second fluids of JP XV (pH 1.2 and 6.8, respectively), and pH 4.0 buffer solution, which were maintained at 37.0 ± 0.5°C. The buff er solution at pH 4.0 was made by mixing the fi rst and the second fluids. The first fluid (pH 1.2) contains 2.0 g of NaCl and 7 ml of HCl in 1000 ml and the second fluid (pH 6.8) contains 250 ml of 0.2 M KH2PO4 and 118 ml of 0.2 M NaOH in 1000 ml. One milliliter of sample solution was taken at appropriate time intervals and assayed by HPLC. Fresh medium was added to replace the sample taken.
The Dissolution characteristics were determined for a 750 mg of AMX and 3 tablets of PST, and for a 250 mg of MTZ and a table of FLG.
Determination of AMX and MTZ by HPLC
The HPLC system consisted of a Shimadzu model LC-10AS pump, SIL-10A auto-injector, SPD-10A UV photometrical detector, and CTO-10A column oven equipped with a Shimadzu model SCL-10A system controller and C-R7A Chromatopac, all from Shimadzu Corporation (Kyoto, Japan). The chromatographic column was an YMC Pack AM312 ODS (150 × 6 mm, i. d.) obtained from YMC Co., Ltd. (Kyoto, Japan). The conditions for the flow rate, the wavelength for determination, and the temperature of the column were 1 mL/min, 254 nm, and 40°C, respectively. The mobile phase used for the determination of AMX and MTZ were acetonitrile-water, 1:19, V/V and 1:9, V/V. The retention times of AMX and MTZ were about 8.6 min and 4.9 min. The concentrations of AMX and MTZ were calculated using calibration curves for the antibiotics. The injection samples for HPLC were prepared by the following procedure: a 30 μl of sample solution was added to 270 μl of the second fluid of JP XV, and then mixed well. A 10 μl of the solution was injected to HPLC.
The simulation of intragastric concentrations
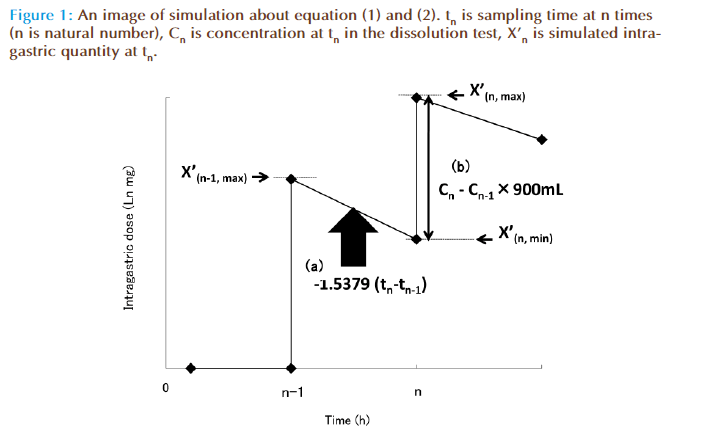
Drug concentrations in stomach were simulated as shown in Figure. 1, using equations (1), and (2) based on the result of dissolution test.
Ln X(n, min) = -1.5379(t n-t n-1) + Ln X(n-1, max) (1)
X(n, max) = X(n, min) + (Cn - Cn-1) × 900 (2)
where Xn is simulated intragastric quantity at tn and X(0, max) = X(0, min) = 0.
where tn is sampling time at n times and n is natural number.
where Cn is concentration at tn in the dissolution test and C0 = 0.
In equation (1) and Figure 1(a) , it showed that as the result of intragastric emptying during tn-tn-1, X(n-1, max) became X(n, min). The intragastric emptying rate of liquid is expressed as first-order kinetics, and a first order rate constant, 1.5379 is the emptying rate constant reported by Camilleri et al [12].
But equation (1) doesn’t include further dissolution quantity between tn and tn-1. Therefore, we corrected it by using equation (2) and Fig. 1 (b). (Cn - Cn-1) × 900 is further dissolution between tn and tn-1 in dissolution test. 900 is the volume of the test media (ml). It is thought that the dissolution quantity of AMX and MTZ in the dissolution test is same as in the stomach because of their solubility. Therefore, we thought that (Cn - Cn-1) × 900 is further dissolution quantity between tn and tn-1 in stomach.
At the end, we converted X into simulated intragastric concentration by multiplied X by 900 ml (stomach volume). In case of AMX, we calculated time above minimum inhibitory concentration (time above MIC, TAM) because AMX is time-dependent antibiotic, and in case of MTZ, we calculated Cmax because it is concentration-dependent antibiotic.
Results and Discussion
Dissolution characteristics of AMX and MTZ
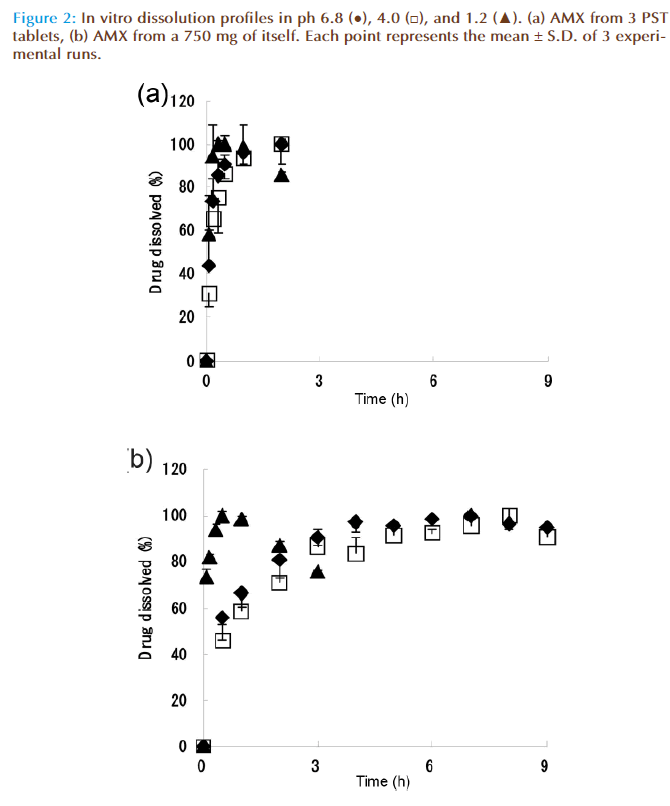
Fig. 2 (a) and (b) show the dissolution characteristics of AMX from 3 PST tablets and a 750 mg of AMX, respectively. In Fig. 2, the dissolution characteristics of pH 4.0 and 6.8 were almost the same. The dissolution rate of AMX at pH 1.2 showed more rapid than those at pH 4.0 and 6.8, which decreased over 30 min due to the degradation of AMX in acidic conditions [13]. On the eradication therapy, PPI was used together. Therefore, the dissolution pattern in stomach was considered to be equivalent to the pattern of the dissolution tests at pH 4.0 and 6.8. We employed the data at pH 4.0 and 6.8. The 100% dissolution time (T100%) of PST at pH 4.0 and 6.8, which were about 20 min, was faster than those of AMX which were about 6 h.
The dissolution characteristics of AMX from a PST tablet and a 250 mg of AMX were confi rmed to be almost same as the results in Fig. 2 (a) and (b) (data not shown).
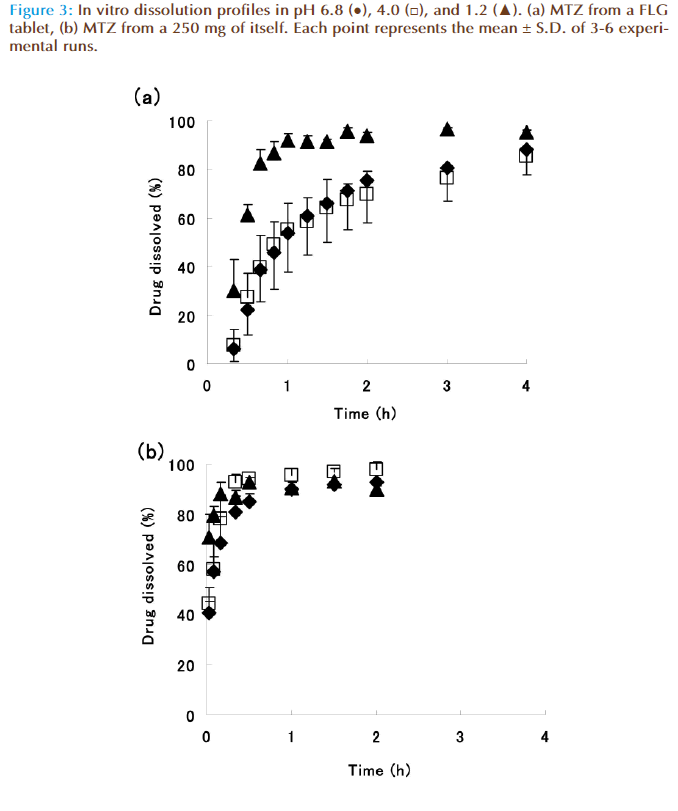
Figures 3 (a) and (b) show dissolution characteristics of MTZ from a FLG tablet and 250 mg of MTZ, respectively. In Fig. 3 (a), the dissolution characteristic of pH 1.2 was rapid those of pH 4.0 and 6.8, and there was no difference between the results at pH 4.0 and 6.8. This difference between pH 1.2 and pH 4.0 and 6.8 might be caused by the sugar-coating layer of the preparation. The dissolution data at pH 4.0 and 6.8 were employed by the same reason in the case of AMX. Th e 100% dissolution time (T100%) of FLG which were about 3 h was later than those of MTZ which were about 30 min.
The simulation of intragastric concentrations
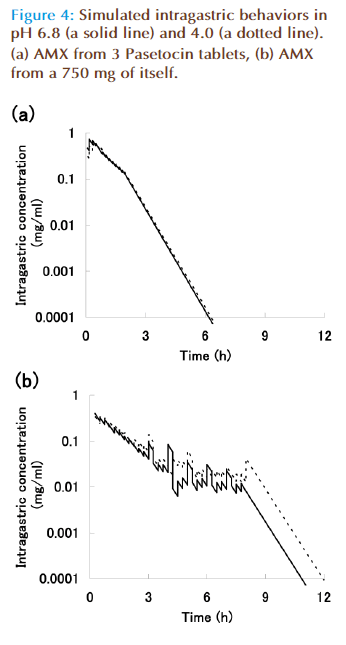
Figures 4 (a) and (b) show intragastric simulated concentrations of AMX from PST and itself, respectively. The point which y is 0.0001 mg/ml is the minimum inhibitor concentration (MIC) of AMX for the bacteria. Solid and dotted lines are intragastric simulated concentration obtained from dissolution test data at pH 6.8 and 4.0, respectively.AMX is time-dependent antibiotic, so an effi cacy depends on a length of time above MIC (TAM). Th erefore, TAM was calculated from the mean value of dissolution test data at pH 6.8 and 4.0. The TAM values of PST and AMX were 6.6 h and 11.4 h, respectively. The value of PST was about three-fifths compared with that of AMX.
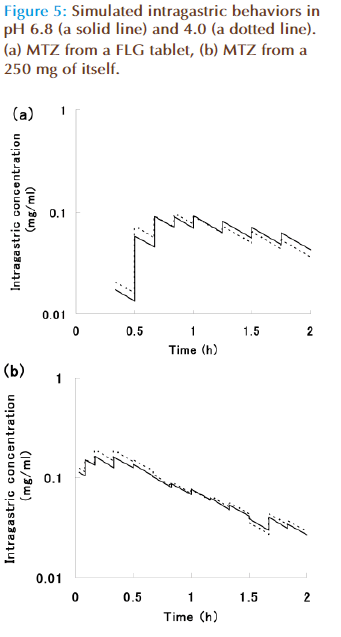
Figures 5 (a) and (b) show simulated intragastric concentrations of FLG and MTZ. MTZ is concentration- dependent antibiotic, so an effi cacy depends on a height of maximum concentration (Cmax). Therefore, Cmax was calculated from the mean values of dissolution test data at pH 6.8 and 4.0. Th e values for FLG and MTZ were 0.095 mg/ml and 0.190 mg/ml, respectively. The Cmax of FLG was about a half compared with that of MTZ.
Conclusion
It was found from our studies that the commercial products, both PST and FLG, were required to improve the drug release rate. In the case of PST, the simulated TAM value (about 6.6 h) was about three-fifths of AMX (11.4 h). The efficacy of AMX depends on a length of TAM. It was found that the effi cacy of PST might be poorer than that of AMX. In the case of FLG, the simulated Cmax value (about 0.095 mg/ml) was a half of MTZ (about 0.190 mg/ml). The efficacy of MTZ depends on a value of Cmax. It was found that the effi cacy of FLG might be poorer than that of MTZ. In addition, our intragastric simulated concentrations were almost same as the clinical data [14].
Consequently, for eradicating H. pylori, preparing more sustained release preparations of AMX and more rapid release preparation of MTZ are considered to be very advantageous in order to decrease doses and to increase the effi cacy over the commercial products.
References
- Ateshkadi A, Lam NP, Johnson CA. Helicobacter pylori and pepticulcer disease. Clin. Pharm. 1993; 12(1): 34-48.
- Axon AT. Helicobacter pylori therapy: effect on peptic ulcer disease.J. Gastroenterol. Hepatol. 1991; 6(2): 131-7.
- Erah PO, Goddard AF, Barrett DA, et al. The stability of amoxicillin,clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J. Antimicrob. Chemother.1997; 39(1): 5-12.
- Katayama H, Nishimura T, Ochi S, et al. Sustained release liquid preparation using sodium alginate for eradication of Helicobacter pylori. Biol. Pharm. Bull. 1999; 22(1): 55-60.
- Megraud F, Brassens-Rabbe MP, Denis F, et al. Seroepidemiology of Campylobacter pylori infection in various populations. J. Clin. Microbiol. 1989; 27(8): 1870-3.
- Holcombe C, Omotara BA, Eldridge J, et al. H. pylori the most common bacterial ingestion in Africa: a random serological study. Am. J. Gastroenterol. 1992; 87(1): 28-30.
- Coghlan JG, Humphries H, Dooley C, et al. Campylobacter pylori and recurrence of duodenal ulcers - a 12 month follow-up study. Lancet. 1987; 2(8568): 1109 - 11.
- Humphreys H, Bourke S, Dooley C, et al. Effect of treatment on Campylobacter pylori in peptic disease: a randomized prospective trial. Gut. 1988; 29(3): 279-83
- Marshall BJ, Goodwin CS, Warren JR, et al. Prospective doubleblind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988; 2(8626-8627): 1437-42.
- Fujioka T, Yoshiiwa A, Okimoto T, et al. Guidelines for the management of Helicobacter pylori infection in Japan: current status and future prospects. J. Gastroenterol. 2007; 42(Suppl 17): 3-6.
- Cooreman MP, Krausgrill P, Hengels KJ. Local gastric and serum amoxicillin concentration after different oral application forms. Antimicrob. Agents. Chemother. 1993; 37(7): 1506-9.
- Camilleri M, Malagelada JR, Brown ML, et al. Relation between antral motility and gastric emptying of solids and liquids in humans. Am. J. Physiol. 1985; 249(5 Pt 1); G580-5.
- Tokumura T, Machida Y. UV absorption method should not be applied for determining amoxicillin in acidic dissolution test medium. Int.J. Pharm. 2001; 228(1-2): 1-4.
- Nakamura M, Spiller R, Barrett D, et al. Gastric juice, gastric tissue and blood antibiotic concentrations following omeprazole, amoxicillin and clarithromycin triple therapy. Helicobacter. 2003; 8(4): 294-9.