Antioxidant and hepatoprotective properties of Indian Sunderban mangrove Bruguiera gymnorrhiza L. leave
- *Corresponding Author:
- Dr. Tapas Kumar Sur
Department of Pharmacology, Institute of Post Graduate Medical Education and Research, 244B, Acharya J. C. Bose Road, Kolkata - 700 020, West Bengal, India.
E-mail: drtapaskumarsur@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Bruguiera gymnorrhiza L. (family Rhizophoraceae) is a true mangrove habitat in Indian Sunderban and traditionally uses for liver disorders. Objectives: The aim was to evaluate antioxidant and hepatoprotective actions of leave extract of B. gymnorrhiza L. Materials and Methods: Hydro‑methanolic extract of mangrove leaves (BR) was standardized using spectrophotometric and high‑performance thin layer chromatography methods. Radical scavenging activities were assessed in different in vitro methods, like 1,1‑diphenyl‑2‑picrylhydrazyl, 2,2’‑azino‑bis‑3‑ethyl benzthiazoline‑6‑sulphonic acid+, superoxides, nitric oxides and hydroxyl radicals. Hepatoprotective efficacy of BR (125 mg/kg and 250 mg/kg, p.o) was measured in D‑galactosamine (GalN) induced (200 mg/kg, i.p) hepatitis in Wistar rats. Silymarin (25 mg/kg, p.o) was used as known hepatoprotective agent. Results: Polyphenols such as gallic acid, quercetin, and coumarin obtained from BR exhibited powerful antioxidant properties. Moreover, it produced dose‑dependent protection against GalN induced hepatitis in rats. It significantly reduced GalN induced elevation of enzymes (alanine transaminase, aspartate aminotransferase, and alkaline phosphatase) in serum and resist oxidative stress marked by lipid peroxides, glutathione, and catalase in hepatic parenchyma. Conclusions: Polyphenols rich B. gymnorrhiza L. leaves ameliorate hepatic tissue injury through its antioxidant effects.
Keywords
Antioxidant, Bruguiera gymnorrhiza, D-galactosamine, liver, mangrove
Introduction
The mangrove flora is a diverse group of salt-tolerant plants growing in tropical and subtropical intertidal estuarine zones and usually categorized into two subgroups, true mangrove, and semi-mangrove according to their living environment.[1] True mangrove is confined to the typical intertidal mangrove habitats where the seawater salinity is usually 17–36%, however, semi-mangrove grow on the landward fringe of the mangrove habitats or in the terrestrial marginal zones that are subjected to irregularly high tides.[2] Bruguiera gymnorrhiza L. (family Rhizophoraceae) is a true mangrove widely distributed in the southern tropical Indian Ocean to tropical Australia.[3] It is the most abundant species in Indian Sunderban.[4] The higher degree of polymorphism might be attributed toward the comfortable growth of B. gymnorrhiza all along Sunderban.[5] It is a salt-tolerant large evergreen tree with elliptic-oblong thick leaves, rough reddish brown bark, short prop-roots, creamy white flowers, ovoid or turbinate single seed berries and viviparous and locally known as Kakra (Hindi), Kankra (Bengali), Banduri (Oriya), Thuddu ponna (Telegu) or Sigapukokandam (Tamil).[6-8] In folk medicine fruits, barks and leaves are commonly used for diarrhea and fever,[6,9] roots and barks are used in the treatment of diabetes,[10] stems are in viral fever[11] while, leaves in burns, intestinal worms, and liver disorders.[6,12-15] Different new and known bioactive constituents such as 3-β-(Z)-coumaroyllupeol and cyclohexylideneacetonitriles were found in hypocotyls,[14,16] dammarane triterpenes (bruguierins) in flowers[17] while, brugunins, and bruguierols in stems.[18] Other derivatives such as amyrins, lupeols, ursolic acid, taraxerol, gymnorhizol, ellagic acid, tannins have been isolated from different parts of this palnt.[2,19-22] Recent studies hypothesized that some of the compounds present in B. gymnorrhiza have potential inhibitory actions on hepatitis B virus[14] and also in HepG2 cells activations,[17] though no experimental evidence is still persist. Hence, this study was undertaken to find out the chemical nature and antioxidant properties of B. gymnorrhiza leaves and its role on D-galactosamine (GalN) induced hepatic tissue injury (resembles to human viral hepatitis) in laboratory animals and also underlying mechanisms in details.
Materials and Methods
Drug and chemicals
Ascorbic acid, bovine serum albumin, catalase, coumarine, 2,2’-Azino-bis-3-ethyl benzthiazoline-6-sulphonic acid (ABTS), 2-deoxyribose, GalN, 1,1-diphenyl-2-picrylhydrazyl (DPPH), gallic acid, glutathione(GSH), malonaldehyde, nitro blue tetrazolium, quercetin, thiobarbituric acid etc., were obtained from Sigma-Aldrich (St. Louis, MO, USA) and precoated silica gel plates (Merck, 60F254, 20 cm × 20 cm) from Germany. Other chemicals and solvents were AR grade and procured from Merck, India. Biological commercial kits were procured from Cogent (Span Diagnostics Ltd., Surat, India).
Plant material
The leaves of B. gymnorrhiza L. (family Rhizophoraceae) was collected from the mangrove forest area of Indian Sunderban, West Bengal, during the postmonsoon period (September to November) and identified by Botanical Survey of India, Howrah. Forest department gave the permission of collection of leaves and the specimen was collected under their supervision. A voucher specimen (DST/537/5G-4/09-04) was deposited in the departmental herbarium. The shed dried powdered leaves were extracted with hydro-methanol (20–80%) in Soxhlet apparatus. Then, it was concentrated and dried (BR) and percentage yield was recorded.
Animals
Wistar male rats (150–160 g) were used in this study. Principles of laboratory animal care guidelines were strictly followed.[23] The animals were maintained under 12:12 h dark: Light cycle and controlled temperature (25 ± 3°C) and fed with food (Provimi, Bangalore, India) and water ad libitum. The experiment was conducted in accordance with the guidelines of Institutional Animal Ethical Committee following due approval (544/c/PO/02/CPCSEA).
Standardization of extracts
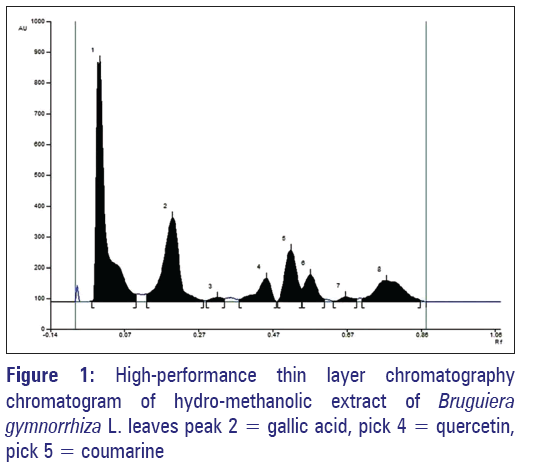
BR was primarily evaluated by standard methods for qualitative analysis of bioactive groups.[24] The total phenolic acids in BR was done according to Folin-Ciocalteu method.[4] Aluminum chloride method was employed to determining the flavonoids present in BR.[4] Finally, the densitometric high-performance thin-layer chromatography (HPTLC) fingerprint of BR was carried out. Gallic acid (3,4,5-trihydroxybenzoic acid), quercetin (5,7,3’,4’-tetrahydroxy flavonol), and coumarin (1,2-benzopyrone) were used as biomarkers. In brief, BR was diluted into 1 mg/ml in methanol and spotted in the form of bands with on a precoated silica gel plates (Merck, 60F254, 20 cm × 20 cm) by Camag Linomat 5. The plates were developed in a mobile solvent system (toluene:ethyl acetate:formic acid = 4.5:3:0.2) for 30 min and scanned at 280 nm suing Camag TLC Scanner 3 (Camag, Linomat 5, USA).[25]
In vitro antioxidant study
The reducing power,[4] DPPH radical scavenging activity,[26] superoxide anion scavenging action,[27] nitric oxide radical formation,[25] hydroxyl radical scavenging by Fenton reaction,[28] and ABTS cation decolorization[29] of BR was done spectrophotometrically following In vitro standard procedures.
In vivo pharmacological study
Acute toxicity
BR was examined for acute oral toxicity for determining the 50% lethal dose following the guideline no. 423 of OECD.[30]
Hepatoprotective activity in rats
Two graded dose of BR (125 mg/kg and 250 mg/kg, p.o) was evaluated on GalN induced hepatic toxicity in rats.[31] The dose has been selected on the basis of 5 point pilot experiment. Silymarin was used as known hepatoprotective agent. Healthy male Wistar rats (150–160 g) were randomized into five following groups (n = 6/group): Group I – normal control (2 ml/kg normal saline); Group II – treated control (2 ml/kg normal saline); Group III – BR treated (125 mg/kg); Group IV – BR treated (250 mg/kg) and Group V – Silymarin (25 mg/kg). The test drug was given orally for 7 consecutive days. On day 5, GalN was administered (200 mg/kg, i.p) to all rats, except normal control. The other treatments were continued as before. After 48 h of hepatotoxin injection, all animals were anesthetized by sodium pentobarbitone (40 mg/kg, i.p) and their blood was collected directly from the heart. Serum alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total protein were estimated using commercial kits.[32] Liver tissues were dissected out, homogenized in cold phosphate buffer (0.1M, pH 7.2) for estimating GSH,[33] catalase,[34] lipid peroxides,[35] and protein.[36]
Statistical analysis
Data have been summarized by routine descriptive statistics. All statistical analysis were performed using the SPSS software, version 17.0 (IBM, USA), differences between and within groups have been assessed for statistical significance by standard parametric and nonparametric tests, as appropriate. P < 0.05 was taken as level of statistical significance in all tests.
Results
Standardization of hydro-methanolic extract of Bruguiera gymnorrhiza leaves
The extractive of BR yields 14.82%. In phytochemical group analysis, BR denotes phenolics, flavonoids, triterpinoids, sterols, and tannins. Further, it shows [Table 1] enrich in phenolics (2.34 μg gallic acid equivalent/mg extract) and flavonoids (5.20 μg quercetin equivalent/mg extract). HPTLC densitometric fingerprint confirms gallic acid (5.90 μg/mg extract), quercetin (8.812 μg/mg extract) and coumarin (14.461 μg/mg extract) in BR [Figure 1].
| Mean±SEM | |
|---|---|
| Extractive value (%) | 14.82±0.047 |
| Quantitative assay | |
| Phenolics (μg GAE/mg extract) | 2.34±0.039 |
| Flavonoids (μg QE/mg extract) | 5.20±0.115 |
| HPTLC densitometric quantification (µg/mg) | |
| Gallic acid | 25.90±0.016 |
| Quercetin | 8.81±0.051 |
| Coumarin | 19.46±0.202 |
| Radical scavenging (IC50) µg/ml | 17.93±0.161 |
| Reducing power | |
| DPPH radical scavenging | 0.355±0.005 |
| NO radical scavenging | 0.305±0.004 |
| SO radical scavenging | 0.356±0.007 |
| HO radical scavenging | 0.311±0.004 |
| ABTS radical scavenging | 0.056±0.0003 |
Table 1: Physicochemical natures and antioxidant properties of Bruguiera gymnorrhiza leaves
In vitro antioxidant study
BR exhibits [Table 1] powerful reducing abilities (17.93 μg/ml), DPPH radical scavenging (0.355 μg/ml), nitric oxide radical inhibitions (0.305 μg/ml), hydroxyl radical scavenging action (0.311 μg/ml), superoxide radical inhibitions (0.356 μg/ml), and ABTS cation diminutions (0.056 μg/ml).
In vivo pharmacological study
The no adverse of BR shows 2.0 g/kg, p.o (limit test) in rats. Three serum liver marker enzymes, ALT, AST, alkaline phosphatise elevates, and protein levels significantly (P < 0.001) reduces within 48 h of GalN injection in rats, while pretreatment of BR shows significant (P < 0.001) and dose dependent protections on these parameters similar to Silymarin, a known hepatoprotective agent [Table 2]. Furthermore, GalN enhances lipid peroxides and diminishes GSH and catalase in hepatic tissues, which reverses significantly (P < 0.001) in pretreatments with BR and Silymarin [Table 3].
| Serum liver function test (mean±SEM) | ||||
|---|---|---|---|---|
| ALT | AST | AKP | Total protein | |
| Normal control | 36±1.06 | 40.3±1.52 | 67±1.86 | 7.41±0.10 |
| GalN control | 141±2.98a,*** | 136.8±2.84a,*** | 182.5±2.66a,*** | 3.18±0.12a,*** |
| GalN + BR (125) | 76.6±2.75b,*** | 79.3±2.49b,*** | 121±3.19b,*** | 4.46±0.12b,** |
| GalN + BR (250) | 68.8±2.27b,*** | 69.1±1.66b,*** | 108.8±3.43b,*** | 5.01±0.11b,*** |
| GalN + SLY (25) | 58.1±2.41b,*** | 60.8±1.92b,*** | 91.1±2.58b,*** | 5.46±0.12b,*** |
aWhen compared with normal control, bWhen compared with GalN control, **P<0.01 and ***P<0.001. GalN: D-galactosamine (200 mg/kg, i.p.), BR: Hydro-methanolic extract of B. gymnorrhiza leaves (125 mg/kg and 250 mg/kg, p.o.), SLY: Silymarine (25 mg/kg, p.o.), n=6; All groups compared using statistical software (SPSS version 17). SEM: Standard error of mean, ALT: Alanine transaminase, AST: Aspartate aminotransferase, AKP: Alkaline phosphatase
Table 2: Effect of Bruguiera gymnorrhiza leaves on liver functions in D-galactosamine hepatic injury in rats
| Liver tissues (mean±SEM) | |||
|---|---|---|---|
| Lipid peroxides | Glutathione | Catalase | |
| (nM MDA/mg | (nM/mg protein) | (μM of H2O2 | |
| protein) | decomposed/ | ||
| min/mg protein) | |||
| Normal control | 1.63±0.03 | 4.31±0.042 | 1.26±0.03 |
| GalN control | 5.47±0.05a,*** | 1.14±0.017a,*** | 0.45±0.26a,*** |
| GalN + BR (125) | 4.42±0.06b,*** | 2.22±0.038b,*** | 0.62±0.02b,** |
| GalN + BR (250) | 4.04±0.06b,*** | 2.35±0.027b,*** | 0.76±0.01b,*** |
| GalN + SLY (25) | 3.50±0.03b,*** | 2.85±0.045b,*** | 0.99±0.03b,*** |
aWhen compared with normal control, bWhen compared with GalN control, **P<0.01 and ***P<0.001. GalN: D-galactosamine (200 mg/kg, i.p.), BR: Hydro-methanolic extract of Bruguiera gymnorrhiza leaves (125 mg/kg and 250 mg/kg, p.o.), SLY: Silymarine (25 mg/kg, p.o.), n=6, All groups compared using statistical software (SPSS version 17), SEM: Standard error of mean
Table 3: Effect of Bruguiera gymnorrhiza on liver tissues in D-galactosamine hepatic injury in rats
Discussion
Hepatic damage involves in most cases of oxidative stresses and is characterized by a progressive evolution from steatosis to chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma.[37] The plant derived antioxidants; particularly polyphenols could be correlated with oxidative stress defense and hepatoprotection.[38] Furthermore, mangrove is rich source of novel compounds with powerful antioxidant properties.[11,25-26] In this study, leaves of B. gymnorrhiza L. (BR) report to have rich source in gallic acid, quercetin, and coumarin and there are considerable data to support their antioxidant and hepatoprotective actions.[39,40] In vitro antioxidant studies show BR has strong reducing abilities and DPPH radical scavenging properties and also has capacities to inhibit generations of ABTS+ radicals and hydroxyl radicals. It is believes that hydroxyl radicals hamper normal liver functions by damaging and breaking DNA strands in hepatic tissues.[41] Moreover, BR has nitric oxide and superoxide radicals scavenging also abilities and thereby it has strong antioxidant properties. Earlier, it was reported that the stems and the roots of this mangrove has potent antioxidant role on polycyclic aromatic hydrocarbon treated tissues.[15]
Hepatotoxin, GalN is mainly concerns either with insufficiency of uridine diphosphate (UDP)-glucose or UDP-galactose that leads to inhibition of energy metabolism on hepatocytes or damaging mitochondrial membrane to release cytochrome C from hepatocytes through oxidative stress.[42,43] These changes resulted in leakage of aminotransferases and alkaline phosphatase enzymes from hepatocytes into serum and considers as sensitive indicators for hepatic injuries. The rises of AST, ALT and alkaline phosphatase in serum are significantly attenuates during BR treatments suggests its stabilizing/protecting role on hepatocyte cell membrane. Further, BR treatment reduces the level of lipid peroxides, most likely due to its strong antioxidant properties. Any reduction in tissue catalase enzyme activity may result in a number of deleterious effects due to the accumulation of hydrogen peroxides in the liver tissues.[37] Additionally, reduced GSH also functions as free radical scavenger and is important in the repair of free radical-induced biological damages.[32] Decreased GSH and catalase levels in GalN induced hepatitis have been considered to be an indicator of oxidative stress.[43] BR resulted in dose dependent and significant restoration of both catalase and GSH in rat liver tissues suggests its potential oxidants defenses. Thus, this study shows that polyphonols present in B. gymnorrhiza L. leaves produce an antioxidant effect and have the potential to treat hepatic injuries.
Conclusion
It may, therefore, suggest that B. gymnorrhiza L. leaves exert a stabilizing effect on hepatocyte cell membrane and promote repair of injure hepatic tissues through its radical scavenging pathways, however, in due course, this study may lead to search more effective hepatoprotective compound(s) from this mangrove for harmonizing therapeutic approaches to hepatic disorders.
Acknowledgements
Authors are grateful to Department of Science and Technology, West Bengal, India for financially supporting this project and to Prof. Amal Kanti Das, Head, Department of Pharmacology, Institute of Post Graduate Medical Education and Research, Kolkata, for permitting use of departmental equipment and facilities.
Financial support and sponsorship
Department of Science and Technology, West Bengal (Project ID: DST/537/5G-4/09).
Conflicts of interest
There are no conflicts of interest.
References
- Wu J, Xiao Q, Xu J, Li MY, Pan JY, Yang MH. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat Prod Rep 2008;25:955-81.
- Zhou ZF, Guo YW. Bioactive natural products from Chinese marine flora and fauna. Acta Pharmacol Sin 2012;33:1159-69.
- Kathiresan K, Rajendran N. Mangrove ecosystems of the Indian Ocean region. Indian J Mar Sci 2005;34:104-13.
- Banerjee D, Chakrabarti S, Hazra AK, Banerjee S, Ray J, Mukherjee A, et al. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr J Biotechnol 2008;7:805-11.
- Dasgupta N, Nandy P, Sengupta C, Das S. RAPD and ISSR marker mediated genetic polymorphism of two mangroves Bruguiera gymnorrhiza and Heritiera fomes from Indian Sundarbans in relation to their sustainability. Physiol Mol Biol Plants 2015;21:375-84.
- Kolkpol U, Miles DH, Payne AM, Chittawong V. Chemical constituents and bioactive compounds from mangrove plants. In: Rahman A, editor. Natural Product Chemistry. Vol. 7. Amsterdam: Elsevier Science; 1990.
- Mandal RN, Naskar KR. Diversity and classification of Indian mangroves: A review. Trop Ecol 2008;49:131-46.
- Zhu Z, Chen J, Zheng HL. Physiological and proteomic characterization of salt tolerance in a mangrove plant, Bruguiera gymnorrhiza (L.) Lam. Tree Physiol 2012;32:1378-88.
- Ahmed F, Shahid IZ, Gain NC, Reza SH, Sadh SK. Antinociceptive and antidiarrhoeal activities of Bruguiera gymnorrhiza. Orient Pharm Exp Med 2007;7:280-5.
- Karimulla B, Kumar K. Antidiabetic and antihyperlipidemic activity of bark of Bruguiera gymnorrhiza on streptozotocin induced diabetic rats. Asian J Pharm Sci Technol 2011;1:4-7.
- Haq M, Sani W, Hossain AB, Taha RM, Monneruzzaman KM. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J Med Plants Res 1999;5:4112-8.
- Vandana KR, Deepthi KB, Yugandhar K, Kiran PS, Saravanakumar A. Anthelmintic and antimicrobial properties of leaves of Bruguiera gymnorrhiza (L.) Lam. Int J Pharm Rev Res 2011;1:1-3.
- Bandaranayake WM. Bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecol Manage 2002;10:421-52.
- Yi XX, Deng JG, Gao CH, Hou XT, Li F, Wang ZP, et al. Four new cyclohexylideneacetonitrile derivatives from the hypocotyl of mangrove (Bruguiera gymnorrhiza). Molecules 201512;20:14565-75.
- Song H, Wang YS, Sun CC, Wang YT, Peng YL, Cheng H. Effects of pyrene on antioxidant systems and lipid peroxidation level in mangrove plants, Bruguiera gymnorrhiza. Ecotoxicology 2012;21:1625-32.
- Yi XX, Gao CH, Long B, Su ZW, Yu L, He BJ. Study on chemical constituents from hypocotyls of mangrove Bruguiera gymnorrhiza. Zhong Yao Cai 2015;38:85-8.
- Homhual S, Bunyapraphatsara N, Kondratyuk T, Herunsalee A, Chaukul W, Pezzuto JM, et al. Bioactive dammarane triterpenes from the mangrove plant Bruguiera gymnorrhiza. J Nat Prod 2006;69:421-4.
- Han L, Huang XS, Sattler I, Fu HZ, Grabley S, Lin WH. Two new constituents from mangrove Bruguiera gymnorrhiza. J Asian Nat Prod Res 2007;9:327-31.
- Gong JX, Shen X, Yao LG, Jiang H, Krohn K, Guo YW. Total synthesis of gymnorrhizol, an unprecedented 15-membered macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Org Lett 2007;9:1715-6.
- Han L, Huang X, Sattler I, Moellmann U, Fu H, Lin W, et al. New aromatic compounds from the marine mangrove Bruguiera gymnorrhiza. Planta Med 2005;71:160-4.
- Sun YQ, Guo YW. Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett 2004;45:5533-5.
- Huang XY, Wang Q, Liu HL, Zhang Y, Xin GR, Shen X, et al. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry 2009;70:2096-100.
- CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol 2003;35:257-74.
- Harbone JB. Phytochemical Methods. 2nd ed. London: Chapman and Hall; 2002.
- Sur TK, Hazra AK, Bhattacharyya D, Hazra A. Antiradical and antidiabetic properties of standardized extract of Sunderban mangrove Rhizophora mucronata. Pharmacogn Mag 2015;11:389-94.
- Banerjee D, Hazra AK, Seal T, Sur TK, Bhattacharyya D, Ray J, et al. Antioxidant and anti-inflammatory activities of different solvent extracts and isolated compounds of Ipomoea pes-caprae (L) sweet of Sunderban mangrove eco-complex. Asian J Chem 2013;25:4997-5000.
- Liu F, Ooi VE, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci 1997;60:763-71.
- Elizabeth K, Rao MN. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;58:237-40.29. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231-7.
- OECD Guideline for the Testing of Chemicals. Acute Oral Toxicity in Animals. OECD/OCDE No. 423; Adopted 21st September, 1998.
- Bhattacharyya D, Pandit S, Jana U, Sen S, Sur TK. Hepatoprotective activity of Adhatoda vasica aqueous leaf extract on D-galactosamine-induced liver damage in rats. Fitoterapia 2005;76:223-5.
- Bhattacharyya D, Pandit S, Mukherjee R, Das N, Sur TK. Hepatoprotective effect of Himoliv, a polyherbal formulation in rats. Indian J Physiol Pharmacol 2003;47:435-40.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67-78.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389-94.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302-10.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-75.
- Lekic N, Canová NK, Horínek A, Farghali H. The involvement of heme oxygenase 1 but not nitric oxide synthase 2 in a hepatoprotective action of quercetin in lipopolysaccharide-induced hepatotoxicity of D-galactosamine sensitized rats. Fitoterapia 2013;87:20-6.
- Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plant Res 2010;4:1318-34.
- Tiwari AK. Imbalance in antioxidant defense and human disease: Multiple approach of natural antioxidant therapy. Curr Sci 2001;80:1179-87.
- Materska M. Quercetin and its derivatives: Chemical structure and bioactivity: A review. Pol J Food Nutr Sci 2008;58:407-13.
- Alkreathy HM, Khan RA, Khan MR, Sahreen S. CCl4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis. BMC Complement Altern Med 2014;14:452.
- Moravcova A, Cervinkova Z, Kucera O, Mezera V, Lotkova H. Antioxidative effect of epigallocatechin gallate against D-galactosamine-induced injury in primary culture of rat hepatocytes. Acta Med (Hradec Kralove) 2014;57:3-8.
- Zhang J, Xu L, Zhang L, Ying Z, Su W, Wang T. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J Nutr 2014;144:1211-8.