Antihistaminic activity of Clitoria ternatea L. roots
- *Corresponding Author:
- Dnyaneshwar J Taur
Department of Pharmacognosy, S.V.P.M’s College of Pharmacy, Malegaon (bk), Baramati, Maharashtra, India.
E-mail: dnyaneshtaur@gmail.com
Date of Received :03-05-2010
Date of Modified :19-07-2010
Date of Accepted :21-07-2010
Available Online :15-02-2011
Abstract
Clonidine, a α2 adrenoreceptor agonist induces dose dependent cata-lepsy in mice, which was inhibited by histamine H1 receptor antagonists but not by H2 receptor antagonist. Clonidine releases histamine from mast cells which is respon-sible for different asthmatic conditions. Clitoria ternatea L. (Family: Fabaceae) is a per-imial twing herb. The roots have anti-inflammatory properties and are useful in severe bronchitis, asthma. In present study ethanol extract of Clitoria ternatea root (ECTR) at doses 100, 125 and 150 mg/kg i.p were evaluated for antihistaminic activity using clonidine and haloperidol induced catalepsy in mice. Finding of investigation showed that chlorpheniramine maleate (CPM) and ECTR inhibit clonidine induced catalepsy significantly P < 0.001 when compare to control group, while CPM and ECTR fail to inhibit haloperidol induced catalepsy. Present study concludes that ECTR possesses antihistaminic activity.
Keywords
ternatea, Clonidine, antihistamine, Chlorpheniramine maleate
Introduction
Clitoria ternatea L. (Family: Fabaceae) a perennial twing herb, steams are terete, more or less pubescent. The roots have a sharp bitter taste and cooling, laxative, diuretic, anthelmintic, anti-inflammatory properties; they are useful in severe bronchitis, asthma and hectic fever [1,2]. The fatty acid content of C.ternatea seeds includes palmitic, stearic, oleic, linoleic, and linolenic acids [3-5]. The seeds also contain a water-soluble mucilage, delphinidin 3, 3’, 5’-triglucoside seful as a food dye [6]; beta-sitosterol [7]. C. ternatea possesses number of pharmacological activities such as nootropic, anxiolytic, antidepressant, anticonvulsant [8], sedative [9], antipyretic, anti-inflammatory and analgesic activities [10]. It enhance the memory, and increase acetylcholine content and acetylcholinesterase activity in rats [11-13]. Objective of present study was to evaluate ethanol extract of Clitoria ternatea root (EECTR) on Clonidine and haloperidol induced catalepsy in mice.
Materials And Methods
Plant material
Roots of C. ternatea were collected in December 2008, from Baramatilocalities, Pune district (Maharashtra), and dried in the shade at room temperature. Dried roots were coarsely powdered in grinder and powder material was kept in air tight container for further study. The plant was identified and authenticated by Prof. R. B. Deshmukh Head Dept. of Botany, Shardabai Pawar Mahila Mahavidyalaya, Baramati; Specimens are deposited, with plant specimen PASR-113.
Extraction
Dried and coarsely powder of C. ternatea roots 500 gm extracted successively with ethanol (95 %) in soxhlet extractor. Extract was concentrated to dryness in rotary evaporator under reduced pressure and dried on water bath to yield ethanol extract of Clitoria ternatea roots (ECTR) 11.27 % w/w.
Animals
Swiss albino mice of either sex weighing 25-30g were housed under standard laboratory conditions, in groups of five. The animals had free access to food and water. The animal ethical committee of the institute approved all the protocols of the study.
Drugs and Chemicals
Clonidine (Unichem, Ltd.); Chlorpheniramine maleate (Alkem, Mumbai), Haloperidol.
Statistical Analysis
The results were reported as mean ± SEM and analyzed for statistical significance using One way ANOVA followed by Dunnett’ test P < 0.05 was considered significant.
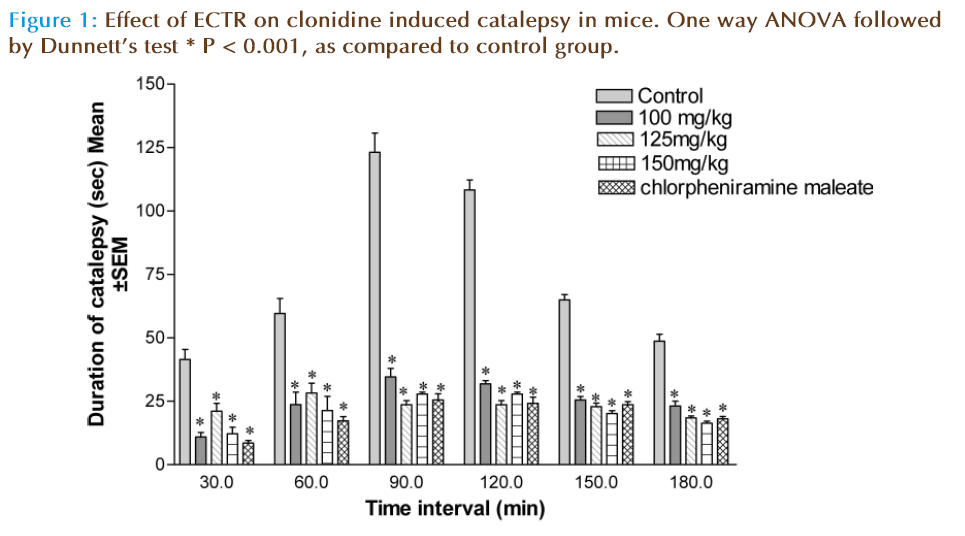
Clonidine-induced catalepsy in mice
Bar test was used to study effect of extracts on clonidine-induced catalepsy, to determine indirect antihistaminic activity. Mice were divided into five groups, five animals in each group. Groups of animal pretreated with (Tween-80 1%, 5 ml/ kg, i.p.), ECTR at doses (100, 125 and 150 mg/ kg i.p) and chlorpheniramine maleate (10 mg/kg, i.p.); received Clonidine (1mg/kg, s.c.) 30 min after treatment. The forepaws of mice were placed on a horizontal bar (1 cm in diameter, 3 cm above the table) and the time required to remove the paws from bar was noted for each animal. The duration of catalepsy was measured at 30, 60, 90, 120, 150 and 180 minute interval after administration of clonidine [14, 15].
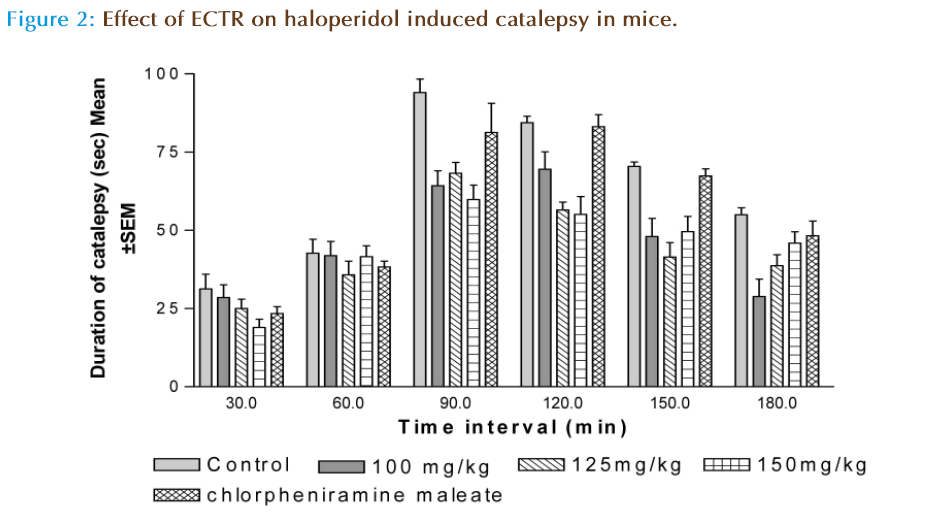
Haloperidol-Induced Catalepsy
The same bar test was used using haloperidol. Groups of animal pretreated with (Tween-80 1%, 5 ml/kg, i.p.), ECTR at doses (100, 125 and 150 mg/kg i.p) and chlorpheniramine maleate (10 mg/kg, i.p.) received Haloperidol (1 mg/kg, i.p.) 30 min after treatment. The forepaws of mice were placed on a horizontal bar (1 cm in diameter, 3 cm above the table) and the time required to remove the paws from bar was noted for each animal. The duration of catalepsy was measured at 30, 60, 90, 120, 150 and 180 minute interval after administration of clonidine [4, 15].
Results
Clonidine-induced catalepsy in mice
Clonidine releases histamine from mast cells which is responsible for different asthmatic conditions. Catalepsy produced by clonidine is mediated by histamine via H1 receptors. The maximum catalepsy was observed after 90 minute of clonidine administration (1 mg/kg, i.p.) in vehicle treated (control) group. Prior treatment with ECTR at doses (100, 125, 150 mg/kg i.p) and Chlorpheniramine maleate (10 mg/kg, i.p.) showed significant (P<0.001) inhibition of clonidine induced catalepsy in dose dependent as shown in (Figure 1).
Haloperidol-Induced Catalepsy
The maximum catalepsy was observed 90 minute after administration of haloperidol (1 mg/kg, i.p.). None of the groups prior treated with ECTR at doses (100, 125, 150 mg/kg i.p) and Chlorpheniramine maleate (10 mg/kg, i.p.) inhibits haloperidol induced catalepsy as shown in (Figure 2).
Discussion
Clonidine, a α2 adrenoreceptor agonist induces dose dependent catalepsy in mice, which was inhibited by histamine H1 receptor antagonists but not by H2 receptor antagonist [16]. Clonidine releases histamine from mast cells which is responsible for different asthmatic conditions [17]. Catalepsy produced by clonidine is mediated by histamine via H1 receptors. In present study it was found that Chlorpheniramine maleate (10 mg/kg, i.p.) and ECTR at doses (100, 125 and 150 mg/kg i.p) inhibit catalepsy in dose dependent manner. Dhanalakshmiet al., (2004) showed that extracts having antihistaminic or mast cell stabilizing effect inhibit clonidine-induced catalepsy [15]. Haloperidol, a non-selective D2 dopamine antagonist induces catalepsy is primarily due to blockade of dopamine receptors in the straiatum. The agents increasing dopamine transmission inhibits haloperidol-induced catalepsy [18]. An antihistaminic drug chlorpheniramine maleate and ECTR fail to inhibit haloperidol induced catalepsy. It indicate that haloperidol induced catalepsy is not mediated by histamine release as antihistaminic drug does not inhibit catalepsy but clonidine induces catalepsy by the release of histamine as it is inhibited by antihistaminic drug. Some plants were investigated for antihistaminic activity inhibit clonidine induced catalepsy in mice Allium sativum and Terminalia belerica [19], Clerodendrum serratum [20], Tamarindus Indica [21], Clitoria ternatea [22], Ficus bengalensis [23], etc.
Present study concluded that the drugs having antihistaminic potential inhibit clonidine induced catalepsy, so ethanol extract of Clitoria ternatea roots possesses antihistaminic activity. Future scope of present investigation is isolate active phytoconstituents which is responsible for antihistaminic activity.
Acknowledgement
The authors are thankful to the Management and Principal Prof. R.N. Patil S.V.P.M’s College of Pharmacy, Malegaon (Bk), Baramati for providing necessary facilities and also to the Prof. R. B. Deshmukh, Dept. of Botany, Shardabai Pawar Mahila Mahavidyalaya, Shardanagar for the authentication of the plant.
References
- Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd ed., Vol-1, International book Distributor, Dehradun, India, 1985.
- Nadkarni AK. Dr. K.M. Nadkarni’s Indian Materia Madica. 3rd edi, Vol-1, Popular Prakashan, Bombay, India, 1992.
- Debnath NB, Chakravarti D, Ghosh A, et al. Fatty acids of Clitoria ternatea seed oils. J of the Institution of Chemists (India). 1975; 47: 253-55.
- Husain S, Devi KS. Fatty acid composition of three plant species: Clitorea ternatea, Mandulea suberosa and Ruta chalapensis. J of the Oil Technologists Association of India. 1998; 30: 162-64.
- Joshi SS, Shrivastava RK, Shrivastava DK. Chemical examination of Clitoria ternatea seeds. J of American Oil and Chemical Society. 1981; 58: 714-15.
- Macedo MLR, Xavier-Filho J. Purification and partial characterization of trypsin inhibitors from seeds of Clitoria ternatea. J of the Science of Food and Agriculture. 1992; 58: 55-58.
- Sinha, A.β-Sitosterol from the seeds of Clitoria ternatea. Cur Sci. 1960; 29: 180- 81.
- Jain NN, Ohal CC, Shroff SK, et al. Clitoria ternatea and the CNS. Pharmac Biochem Behav. 2003; 75: 529–36
- Kulkarni C, Pattanshetty JR, Amruthraj G. Effect of alcoholic extract of Clitoria ternatea Linn. on central nervous system in rodents. Ind J Exp Biol.1988; 26: 957–60.
- Parimaladevi B, Boominathan R, Mandal SC. Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia. 2003; 74: 345–49.
- Rai KS, Murthy KD, Karanth KS, et al. Clitoria ternatea Linn root extract treatment during growth spurt period enhances learning and memory in rats. Ind J Physiol Pharmac. 2001; 45: 305–13.
- Rai KS, Murthy KD, Karanth KS, et al. Clitoria ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia. 2002; 73: 685–89.
- Taranalli AD, Cheeramkuczhi TC. Influence of Clitoria ternatea on memory and central cholinergic activity in rats. Pharm Biol. 2000; 38: 51–56
- Ferre S, Guix T, Prat G, et al. Is experimental catalepsy properly measured? Pharmac Biochem Behav 1990; 35: 753-57.
- Dhanalakshmi S, Khaserao SS, Kasture SB. Effect of ethanolic extract of some anti-asthmatic herbs on clonoidine and haloperidol- induced catalepsy in mice. OPEM. 2004; 4:1-5.
- Jadhav JH, Balsara JJ, Chandorkar AG. Involvement of histaminergic mechanisms in the cataleptogenic effect of clonidine in the mice. J Pharm Pharmacol. 1983; 35: 671-73.
- Lakdawala AD, Dadkar NK, Dohadwala AN. Action of clonidine on mast cells of rats. J Pharm Pharmacol. 1980; 32: 790- 91.
- Sanberg PR. Haloperidol-induced catalepsy is mediated by postsynaptic dopamine receptors. Nature. 1980; 284: 472-73.
- Vyas BA, Vyas RB. Effect of ethanolic extracts of Allium sativum and Terminalia belerica on clonidine-induced mast cell degranulation and clonidine and haloperidol-induced catalepsy in mice. Int J Pharm Res. 2009; 1(1): 41-44
- Bhujbal SS, Kewatkar SM, Dinesh Kumar, et al. In vivo and in vitro antiasthmatic studies of Clerodendrum serratum linn. Pharmacologyonline. 2009; 2: 745-52.
- Tayade PM, Ghaisas MM, Jagtap SA, et al. Anti-asthmatic activity of methanolic extract of leaves of Tamarindus Indica Linn. J Pharm Res. 2009; 2(5): 944-47.
- Taur DJ, Patil RY, Khalate AH. Phytochemical investigation and evaluation of Clitoria ternatea seeds extracts on clonidine induced catalepsy in mice. Pharmacologyonline. 2009; 3: 215-20.
- Taur DJ, Nirmal SA, Patil RY. Effect of various extracts of Ficus bengalensis bark on clonidine and haloperidol-induced catalepsy in mice. Pharmacologyonline. 2007; 3: 470-77.