Antihepatotoxic effect of Marrubium vulgare and Withania somnifera extracts on carbon tetrachloride-induced hepatotoxicity in rats
- *Corresponding Author:
- Essam Abdel-Sattar
Department of Natural Products, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
E-mail: abdelsattar@yahoo.com
Date of Received: 03-04-2010
Date of Modified: 25-06-2010
Date of Accepted: 21-08-2010
Available Online: 15-11-2010
Abstract
Marrubium vulgare and Withania somnifera are used in folk medicine of several countries. Many researches showed that they are used for the treatment of variety of diseases due to their antioxidant effects. The present aim of this study was to evaluate the antihepatotoxic and antioxidant activities of the both extracts against carbon tetrachloride (CCl4)-induced hepatic damage in rats. Both extracts were given orally in a dose of 500 mg/kg/day for 4 weeks along with CCl4 started at the 7th week of induction of hepatotoxicity. The antihepatotoxic activity was assessed by measur-ing aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), glutathione peroxidase (GPx), glutathione reduct-ase (GR), glutathione-S-transferase (GST), reduced glutathione (GSH), tissue content and malondialdehyde (MDA) as well as histopathological examination. Both extracts showed a significant antihepatotoxic effect by reducing significantly the levels of AST, ALT and LDH. However, ALP levels were decreased non-significantly. Regarding the antioxidant activity, they exhibited significant effects by increasing the GPx, GR and GST activities with increased GSH tissue contents and decreased production of MDA level. Furthermore, both extracts alleviated histopathological changes in rats' liver treated with CCl4. M. vulgare and W. somnifera protect the rats' liver against CCl4-induced hepatotoxicity. This effect may be attributed, at least in part, to the antioxidant activities of these extracts.
Keywords
Marrubium vulgare, Withania somnifera, antihepatotoxic, carbon tetrachloride (CCl4), antioxidant.
Introduction
Liver damage is one of the most serious diseases which has accompanied the adoption of modern food styles as well as exposure to many environmental pollutants and intensive intake of medications. Various xenobiotics are known to cause hepatotoxicity, one among them is carbon tetrachloride (CCl4) that may cause lipid peroxidation [1,2].
Oriental herbal medicines have recently attracted the interest of modern scientific communities as alternative therapy. There has been a sharp upward trend in the use of phytomedicines over the last decades in Europe and USA [3,4]. Marrubium vulgare and Withania somnifera are some of medicinal plants that are grown and distributed in Saudi Arabia and used in folk medicine of several other countries. M. vulgare is used for the treatment of variety of diseases, including inflammatory, gastroenterical and respiratory disorders [5]. On the other hand, the root of W. somnifera, known as Indian ginseng (Ashwagandha), has been described in Ayurvedic folk medicine to have potent aphrodisiac, sedative, energy-enhancing tonic properties and in geriatric problems [6]. Many investigators have reported that W. somnifera possesses anabolic, antiserotogenic and anticancer activities. Moreover, it is beneficial in the treatment of arthritis, geriatric problems, stress, and male sexual dysfunctions. It also has adaptogenic, cardiotropic, cardioprotective, and anticoagulant properties [7]. W. somnifera has been shown to inhibit lipid peroxidation (LP) in stress-induced animals [8]. The proved activities of W. somnifera as adaptogenic, anti-inflammatory, antioxidant, anti-platelet, antihypertensive, hypoglycemic and hypolipidemic eff ects may contribute to its cardioprotective properties [9].
The current study was designed to investigate the potential antihepatotoxic eff ect of aerial parts of M. vulgare and W. somnifera in CCl4-induced hepatotoxicity and liver damage in rats.
Experimental
Plant material and extraction
The aerial parts of M. vulgare L. and W. somnifera (L.) were collected from Wadi Kama, Al-Taif governorate, Saudi Arabia, in February 2009 and were dried in shade. A specimen was deposited in the herbarium of college of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia (M. vulgare #. MV1105; W. somnifera # WS1154) and were identified by staff of department of biology, Faculty of Science, King Abdulaziz University. The dried aerial parts of each plant (500 g) were extracted with methanol using Ultra turrax homogenizer (3 X 2000 mL). The solvent was distilled off under reduced pressure and the dried methanolic extract was kept at 4°C.
Chemicals
Carboxymethylcellulose sodium (CMC-Na) was purchased from Acros Organics (NJ, USA). Liquid paraffin, carbon tetrachloride and heparin sodium were purchased from Merck (Dramstadt, Germany). All other biodiagnostic kits were purchased from Diagnostic and Research Reagents (Giza, Egypt).
Animals and their treatment
Male Wister rats, weighing 200-250g were used in this study in accordance with the guidelines of the Biochemical and Research Ethical Committee at King Abdulaziz University, Jeddah, Saudi Arabia. Animals were purchased from the animal house of King Fahed Medical Research Center, King Abdulaziz University and were housed in a well-ventilated, temperature-controlled room at 22 ± 3°C with 12 h light dark cycle. Food consisted of normal rat chow and water was provided ad libitum. Care was taken to avoid stressful conditions. All experimental procedures were performed between 8-10 a.m.
Group I (normal control, n=8), received liquid paraffin (1ml/kg b.w., s.c.) each other day for 10 weeks. Starting from day 43 (7th week), rats received CMC-Na; 1% (1 ml/200 g body weight/ day) orally by intragastric tube for 4 weeks. Hepatotoxicity in rats of groups (II - V) was induced by s.c. injection of CCl4 dissolved in liquid paraffin (30% solution), in a dose of 1ml/kg b.w. for 6 weeks [10]. Animal treatments with vehicle, silymarin or plant extracts started at the 7th week of induction of hepatotoxicity and continued for 4 weeks along with CCl4 injection. Group II (CCl4-treated rats, n=8), received a solution of 1% CMC-Na orally in the same previous dose for 4 weeks and served as a negative control group along with CCl4. Group III (Silymarin-treated group, n=8), received silymarin in a dose of 150 mg/kg/day suspended in 1% CMC-Na by oral gavage once daily for 4 weeks along with CCl4 [11]. Groups IV and V (extractstreated groups n=8 each), received M. vulgare and W. somnifera Extracts, respectively, suspended in 1%CMC-Na by oral gavage in a dose of 500 mg/ kg/day for 4 weeks along with CCl4. Doses of both extracts were chosen according to unpublished preliminary experiments conducted by our research group (see acknowledgement).
At the end of the experiment, twenty four hours after dosing of vehicle, CCl4 or plant extracts, blood samples were collected from the orbital sinus. Serum was separated by centrifugation at 3500 rpm and kept under -70°C for determination of liver enzymes. Animals were anesthetized with diethyl ether and sacrificed by cervical dislocation for separation of the liver. Livers were dissected out, divided into two parts. One part was kept in liquid nitrogen for determination of antioxidant status and the other part was immediately fixed in buff - ered formalin 10% and was used for histopathological examination.
Assay of liver function
The biochemical parameters such as AST, ALT, ALP and LDH were determined using the commercially available kits according to the manufacturer’s instructions.
Assay of liver antioxidant status
Liver superoxide dismutase (SOD) activity was determined according to the method described by Sun and Zigman [12], while, the activities of hepatic glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione S-transferase (GST) activity were determined according to Mohandas et al. [13]. Reduced glutathione (GSH) tissue content was measured according to the method described by Moron et al. [14], while LP products were determined by measuring malondialdehyde (MDA) content in liver tissue homogenates according to the method of Uchiyama and Mihara [15]. Values are expressed as nmol/g protein.
Histopathological analysis
For histological studies, liver tissues were fixed with 10% phosphate-buff ered neutral formalin, dehydrated in graded (50–100%) alcohol and embedded in paraffin. Th in sections were cut and stained with hematoxylin and eosin stain for microscopic assessment. The initial examination was qualitative, with the purpose of determining histopathological lesions in liver tissue.
Statistical analysis
Data are expressed as mean ± standard error (S.E.) of the mean. Unless otherwise indicated, statistical analyses were performed using one-way analysis of variance (ANOVA). If the overall F-value was found statistically significant (p<0.05), further comparisons among groups were made according post hoc Tuckey's test. All statistical analyses were performed using GraphPad InStat 3 (GraphPad Software, Inc. La Jolla, CA, USA) software.
Results
Evaluation of liver function
Results in table (1) showed that s.c. injection of CCl4 induced a significant increase in serum level of liver enzymes at the end of the experiment. Administration of silymarin for 4 weeks induced significant reduction in the blood levels of AST, ALT and LDH, but did not significantly aff ect the ALP levels. M. vulgare extract significantly reduced the blood levels of the AST, ALT and LDH (p<0.05), compared to the values of CCl4-treated group. Although, it has also decreased ALP level, this reduction was not significant. Administration of W. somnifera extract significantly reduced AST, ALT, ALP and LDH serum levels compared with CCl4-treated rat values.
| AST (U/L) | ALT (U/L) | ALP (U/L) | LDH (U/L) | |
|---|---|---|---|---|
| Normal control | 106.25 ± 12.25 | 28.53 ± 2.16 | 216.97 ± 28.31 | 198 ± 20.30 |
| CCl4 control | 204.44 ± 9.53* | 234.39 ± 19.76* | 509.02 ± 32.44* | 434 ± 31.22* |
| Silymarin + CCl4 | 138.90 ± 17.12** | 114.13 ± 17.34** | 463.92 ± 37.81 | 210 ± 22.13** |
| M. vulgare + CCl4 | 100.18 ± 22.00** | 105.01 ± 16.45** | 373.17 ± 32.85 | 242 ± 20.19** |
| W. somnifera + CCl4 | 108.40 ± 10.93** | 97.25 ± 19.14** | 281.05 ± 22.20** | 260 ± 21.22** |
The values are expressed as the mean ± SE of the mean of 8 rats.
* Signifi cantly different from the values of the normal rats at p <0.05.
** Signifi cantly different from the control values of CCl4-induced hepatotoxic rats at p <0.05.
Table 1: The effect of M. vulgare and W. somnifera extracts on CCl4-treated induced alterations in serum hepatic enzymes including aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH)
Evaluation of the antioxidant activity
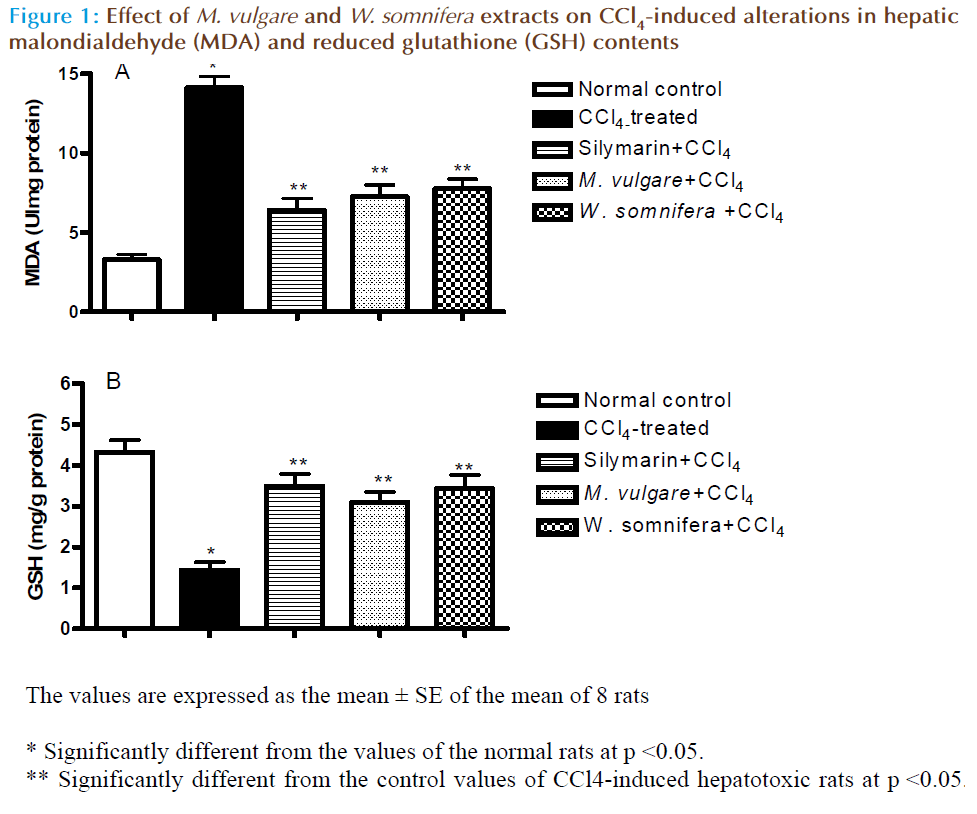
Results presented in table (2) and figure (1) showed that s.c. injection of CCl4-induced significant reduction in the enzyme activities of GPx , GR and GST with a decreased level of GSH content. On other hand, it increased the MDA level in liver tissues compared to normal control values. However, CCl4 did not aff ect the activity of SOD in liver tissues. Silymarin significantly increased the GPx, GR and GST activities with increased GSH tissue contents and decreased MDA level in tissue homogenate with no eff ect on the SOD activity. Similarly, M. vulgare and W. somnifera extracts exerted similar significant eff ect on oxidant status comparable to that observed with silymarin, with no significant eff ect on SOD activity compared to CCl4-treated rat values.
| SOD(U/mg protein) | GPx (nmol/mg protein) | GR (U/mg protein) | GST (nmol/ mg protein) | |
|---|---|---|---|---|
| Normal control | 45.8675 ± 14.03 | 123.56 ± 40.80 | 84.82 ± 8.62 | 1004.8 ± 72.3 |
| CCl4 control | 63.4075 ± 13.68 | 46 .74 ± 13.10* | 34.53 ± 3.24* | 353.5 ± 59.4 * |
| Silymarin + CCl4 | 61.4925 ± 7.24 | 99.29 ± 47.87** | 44.10 ± 4.30** | 441.3 ± 39.5 * |
| M. vulgare + CCl4 | 57.9425 ± 16.74 | 95.396 ± 12.01** | 46.36 ± 4.42** | 864.6 ± 51.4** |
| W. somnifera + CCl4 | 63.142 ± 4.06 | 91.03 ± 17.19** | 47.36 ± 4.52** | 754.7 ± 46.3** |
The values are expressed as the mean ± SE of the mean of 8 rats.
* Signifi cantly different from the values of the normal rats at p <0.05.
** Signifi cantly different from the control values of CCl4-induced hepatotoxic rats at p <0.05.
Table 2: The effect of M. vulgare and W. somnifera extracts on CCl4-induced hepatic alterations in superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST)
Evaluation of histopathological changes
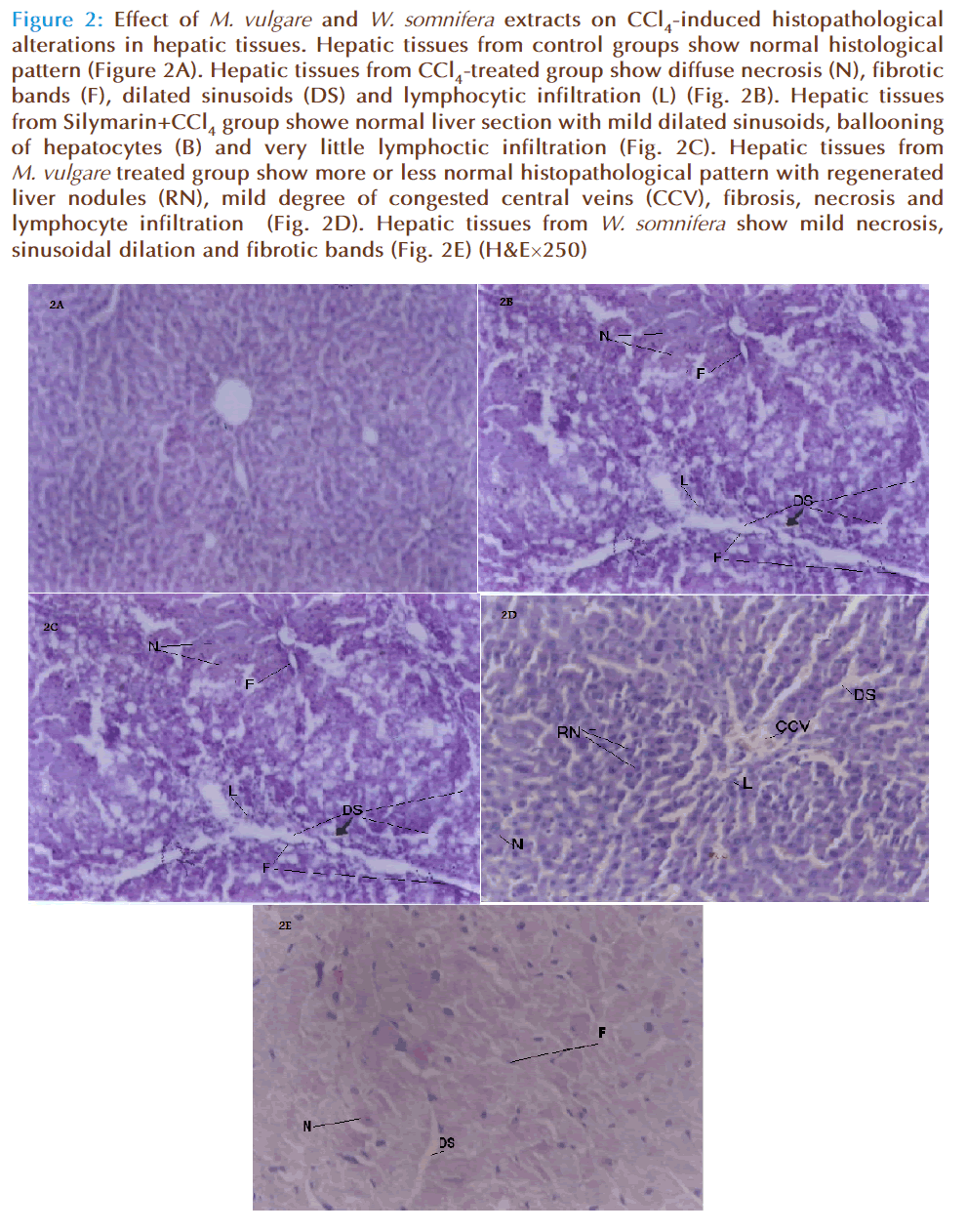
Histopathological examination of the liver sections from normal rats showed normal parenchymal architecture; no significant lesions were observed (Fig. 2A). In CCl4-treated group, diff use central and peripheral necrosis, destruction of the lobular architecture and the formation of septa with sinusoidal dilation was seen (Fig. 2B). Silymarin+ CCl4-treated group showed normal liver section with mild dilated blood sinusoids and very little effect on liver tissues (Fig. 2C). In addition, treatment with M. vulgare and W. somnifera showed more or less normal histopathological pattern with regenerated liver nodules and mild degree of necrosis and lymphocyte infiltration (Fig. 2 D and Fig. 2E).
Figure 2: Effect of M. vulgare and W. somnifera extracts on CCl4-induced histopathological alterations in hepatic tissues. Hepatic tissues from control groups show normal histological pattern (Figure 2A). Hepatic tissues from CCl4-treated group show diffuse necrosis (N), fibrotic bands (F), dilated sinusoids (DS) and lymphocytic infiltration (L) (Fig. 2B). Hepatic tissues from Silymarin+CCl4 group showe normal liver section with mild dilated sinusoids, ballooning of hepatocytes (B) and very little lymphoctic infiltration (Fig. 2C). Hepatic tissues from M. vulgare treated group show more or less normal histopathological pattern with regenerated liver nodules (RN), mild degree of congested central veins (CCV), fibrosis, necrosis and lymphocyte infiltration (Fig. 2D). Hepatic tissues from W. somnifera show mild necrosis, sinusoidal dilation and fibrotic bands (Fig. 2E) (H&E×250)
Discussion
Liver damage is always associated with cellular necrosis, increase in tissue LP and depletion of reduced liver glutathione. In addition, elevated levels of hepatic serum enzymes are indicative of cellular leakage [16]. Among xenobiotics, CCl4 represents the main cause of acute liver injury through its bioactivation to trichloromethyl free radicals that cause LP and produces hepatocellular damage [17,18]. In our study, CCl4 induced severe liver damage as evidenced by the significant elevation of serum levels of ALT, AST, ALP and LDH that indicates the severity of liver injury [19]. These eff ects were coupled with a marked hepatic oxidative stress as well as histopathological changes indicating liver injury. Oxidative stress was evidenced by decreased GSH liver tissue content as well as decreased SOD, GPx, GR and GST activities coupled with the increased production of MDA. It is well known that GSH is a major non-enzymatic antioxidant and plays an important role in cellular defense, which is a crucial determinant of tissue susceptibility to oxidative damage [20,21]. GSH depletion occurs as a consequence of CCl4-induced toxicity. During the radical stress, GSH is oxidized by GPx to oxidised glutathione, which can then be reduced back to GSH by GR. Reduced glutathione is also a cofactor for GST, primarily involved in the detoxification of electrophilic xenobiotics via catalysing the formation of GSH-electrophile conjugate [22-24]. In addition, SOD catalyses the dismutation of superoxide anion to H2O2 and O2. Because H2O2 is still harmful to cells, catalase and GPx further catalyse the decomposition of H2O2 to water [24]. The increase in MDA levels, as evident in our study, suggests enhanced LP leading to tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals [25].
Silymarin, a mixture of flavonolignanes from milk thistle (Silybum marianum L.), is a hepatoprotective herbal medicine with potent antioxidant activity and has been used as a positive control drug in similar animal models [26-28]. Treatment with M. vulgare and W. somnifera improved the liver function, an eff ect that was evidenced by the significant reduction in AST, ALT and LDH. Th is improvement of liver function, in both M. vulgare and W. somnifera treated groups, was also supported by histopathological examination which revealed amelioration of pathological changes observed in CCl4-treated group. The possible mechanism of the antihepatotoxic eff ect of both extracts may be, in part, attributed to their antioxidant activities. Th is eff ect was evidenced by the significant increase in the GSH tissue contents and decreased GPx activity in both M. vulgare and W. somnifera treated groups. Complementing our findings, earlier studies have reported that both extracts may have antioxidant activities. M. vulgare leaves have been reported to be rich in phenolic compounds. Four phenylpropanoid glycosides (verbascoside, forsythoside, arenarioside and ballotetroside) have been isolated and characterized by Martin-Nizard et al. [29] and these compounds were previously served as free radical scavengers [30]. In addition, the antioxidant eff ects of W. somnifera depend on the presence of steroidal lactones, withanolides, which are the main active constituents (2.8%) [8,31]. Moreover, the herb root was found to aff ord antioxidants, and therefore the herb root was used to treat various diseases including stress, anxiety, insomnia arthritis and neurodegeneration [32-35].
In conclusion, our results support the possible antihepatotoxic eff ect of both M. vulgare and W. somnifera extracts against CCl4-induced hepatotoxicity in rats. Th is antihepatotoxic eff ect may be attributed partially to their antioxidant activity.
Conflict of Interest
The authors declare that there are no conflicts of interest
Acknowledgement
We thank Mr. Islam Farouk, Department of Pharmacology and Toxicology, Faculty of Pharmacy, King Abdulaziz University, for his technical assistance. Th is work was supported in part by research grants from the Deanship of Scientific Research, King Abdulaziz University, Saudi Arabia (Project No. 046 / 428).
References
- Kodavanti PR, Joshi UM, Young YA, Meydrech EF, Mehendale HM. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicology and Pathology. 1989; 17: 494–505.
- Demirdag K, Bakcecioglu IH, Ozercan IH, Ozden M, Yilmaz S, Kalkan A. Role of L-carnitine in the prevention of acute liver damage induced by carbon tetrachloride in rats. Journal of Gastroenterology and Hepatology. 2004; 19: 333–338.
- Ernst E. The role of complementary and alternative medicine. British Medical Journal. 2000; 321: 1133–1135.
- Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, Kaptchuk TJ, Eisenberg DM. Long-term trends in the use of complementary and alternative medical therapies in the United States. Annals of internal medicine. 2001; 135: 262–268.
- Salinas GMM, Guerra MCR, Villareal JV, Carden´as BDM, Montes PB, Fernand´ez SS. Bacterial activity of organic extracts from Flourensia cernua against strains of Mycobacterium tuberculosis. Archives of Medical Research. 2005; 37: 45–49.
- Williamson EM. Major Herbs of Ayurveda; Churchill Livingstone: London, UK; 2002, pp. 322-323.
- Misra LC, Singh BB, Degenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alternative medicine review. 2000; 5: 334–346.
- Dhuley JN. Effect of ashwagandha on lipid peroxidation in stressinduced animals. Journal of Ethanopharmacology. 1998; 60:173–178.
- Ojha SK, Arya DS. Withania somnifera Dunal (Ashwagandha): A Promising Remedy for Cardiovascular Diseases. World Journal of Medical Sciences. 2009; 4: 156-158.
- Bhandarkar MR, Khan A. Antihepatotoxic effect of Nymphaea stella willd., against carbon tetrachloride-induced hepatic damage in albino rats. Journal of Ethanopharmacology. 2004; 91: 61-64
- Balamurugan M. Restoration of histoarchitecture in the paracetamol- induced liver damaged rat by earthworm extract, Lampito mauritii (Kinberg). European review for medical and pharmacological sciences. 2007; 11: 407-411
- Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Analytical biochemistry. 1978; 247: 81–89.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Research. 1984; 44: 5086–5091.
- Moron MJ, Diperre JW, Mannerv KB. Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lungs and liver. Biochemica et Biophysica Acta. 1979; 582: 67–71.
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical biochemistry. 1978; 86: 271-278.
- Drotman RB, Lawhorn GT. Serum enzymes are indicators of chemical induced liver damage. Drug and Chemical Toxicology. 1978; 1: 163–171.
- Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Seminars in liver disease. 1990; 10: 56–65.
- Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Molecular aspects of medicine. 2000; 21: 49–98.
- Lin SC, Yao CJ, Lin CC, Lin YH. Hepatoprotective activity of Taiwan folk medicine: Eclipta prostrate Linn. against various hapatotoxins induced acute hapatotoxicity. Phototherapy Research. 1996; 10: 483-490.
- Prescott LF. Glutathione: A protective mechanism against hepatotoxicity. Biochemical Society transactions. 1982; 10: 84-85.
- Meister A, Anderson M E. Glutathione. Annual Reviews in Biochemistry. 1983; 52: 711–760.
- Trush MA, Mimnaugh EG, Gram TE. Activation of pharmacologic agents to radical intermediates: Implications for the role of free radicals in drug action and toxicity. Biochemical pharmacology. 1982; 31: 3335-3336.
- James RC, Goodman DR, Harbison RD. Hepatic glutathione and hepatotoxicity: Changes induced by selected narcotics. The Journal of pharmacology and experimental therapeutics. 1982; 22: 708-714.
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual review of pharmacology and toxicology. 2005; 45: 51–88.
- Souza MF, Rao VSN, Silveira ER. Inhibition of lipid peroxidation by ternatin, a tetramethoxyflavone from Egletes viscosa L. Phytomedicine. 1997; 4: 25–29.
- Girish SA, Sudhir GW, Avinash KD. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. Journal of Ethanopharmacology. 2004; 90: 229–232
- Wills PJ, Asha VV. Protective effect of Lygodium flexuosum (L.) Sw. (Lygodiaceae) against D-galactosamine induced liver injury in rats. Journal of Ethanopharmacology. 2006; 108: 116–123.
- Tung YT, Wu JH, Huang CC, Peng HC, Chen YL, Yang SC, Chang ST. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food and Chemical Toxicology. 2009; 47: 1385–1392.
- Martin-Nizard F, Sahpaz S, Furman C, Fruchart JC, Duriez P, Bailleul F. Natural phenylpropanoids protect endothelial cells against oxidized LDLinduced cytotoxicity. Planta Medica. 2003; 69: 207–211.
- Martin-Nizard F, Sahpaz S, Kandoussi A, Carpentier M, Fruchart JC, Duriez P, Bailleul F. Natural phenylpropanoids inhibit lipoprotein-induced endothelin-1 secretion by endothelial cells. Journal of Pharmacy and Pharmacology. 2004; 56: 1607–1611.
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. Journal of Ethanopharmacology. 2000; 71:23-43.
- Russo A, Izzo AA, Cardile V, Borrelli F, Vanella A. Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine. 2001; 8: 125– 132.
- Gupta SK, Dua A, Vohra BP. Withania somnifera (Ashwagandha) attenuates antioxidant defense in aged spinal cord and inhibits copper induced lipid peroxidation and protein oxidative modifications. Drug Metabolism and Drug Interactions. 2003; 19: 211–222.
- Kaur P, Sharma M, Mathur S, Tiwari M, Divekar HM, Kumar R, Srivastava KK, Chandra R. Effect of 1-oxo-5beta, 6beta-epoxy-witha- 2-ene-27-ethoxyolide isolated from the roots of Withania somnifera on stress indices in Wistar rats. Journal of Alternative and Complementary Medicine. 2003; 9: 897–907.
- RajaSankar S, Manivasagam T, Sankar V, Prakash S, Muthusamy R, Krishnamurti A, Surendran S. Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson's disease model mouse. Journal of Ethanopharmacology. 2009; 125: 369-373.