Antiarthritic activity of a polyherbal formulation against Freund's complete adjuvant induced arthritis in Female Wistar rats
- *Corresponding Author:
- Mr. R. Ramesh Petchi
Department of Pharmacology, Vasavi Institute of Pharmaceutical Sciences, Peddapalli, Opposite to Bhakrapeta Railway Station, Kadapa, Andhra Pradesh, India.
E-mail: rameshpetchi28@gmail.com
Abstract
Objectives: To formulate a polyherbal formulation and evaluate its antiarthritic activity against Freund’s complete adjuvant induced arthritis in Female Wistar rats. Materials and Methods: Glycosmis pentaphylla, Tridax procumbens, and Mangifera indica are well‑known plants available throughout India and they are commonly used for the treatment of various diseases including arthritis. The polyherbal formulation was formulated using the ethanol extracts of the stem bark of G. pentaphylla, whole plant of T. procumbens, and leaves of M. indica. The polyherbal formulation contains the ethanol extracts of G. pentaphylla, T. procumbens, and M. indica in the ratio of 2:2:1. The quality of the finished product was evaluated as per the World Health Organization’s guidelines for the quality control of herbal materials. Arthritis was induced in female Wistar rats using Freund’s complete adjuvant (FCA), and the antiarthritic effect of polyherbal formulation was studied at doses of 250 and 500 mg/kg. The effects were compared with those of indomethacin (10 mg/kg). At the end of the study, blood samples were collected for biochemical and hematological analysis. The radiological examination was carried out before terminating the study. Results: Polyherbal formulation showed significant antiarthritic activity at 250 and 500 mg/kg, respectively, and this effect was comparable with that of indomethacin. The antiarthritic activity of polyherbal formulation is supported by biochemical and hematological analysis. Conclusion: The polyherbal formulation showed signinicant antiarthritic activity against FCA‑induced arthritis in female Wistar rats.
Keywords
Arthritis, Fingerprint analysis, Glycosmis pentaphylla, Mangifera indica, Tridax procumbens
Introduction
Arthritis is a painful swelling of joints and it is a common disease affecting large population. Osteoarthritis and rheumatoid arthritis are most common. Osteoarthritis is a degenerative joint disease occurring chiefly in older people and rheumatoid arthritis is an autoimmune disorder of unknown etiology.[1] In complementary and alternative medicines such as Ayurveda (herbs) and acupuncture are most commonly used for the treatment of many systemic disorders.[2] Chopra et al., reported around 68% patients with chronic rheumatic disorders has sought relief using alternative system of medicine and demonstrated the clinical efficacy of herbal formulation for the treatment of osteoarthritis of the knees.[3,4]
Many herbs and herbal medicines have been used since time immemorial to cure many disorders/diseases, including arthritis. However, the scientific basis for such uses is not completely established. Therefore it is necessary to screen various herbs and natural products for their pharmacological properties. Plants are richest sources of pharmaceutical lead molecules and their contribution in drug discovery process is remarkable. Objective of the present study is to formulate a polyherbal formulation (PHF) and evaluate its antiarthritic potential in animals. The PHF was formulated using the herbs which have known antiarthritic effects at particular ratio to enhance the pharmacological activity of individual herb and reduce the dose of single plant extract. In Ayurveda two principles are used for drug formulation viz., single herb or more than one herb i.e., PHF and this was highlighted in ‘Sarangdhar Samhita’ dated centuries ago in 1300 A. D. Moreover PHF used to archive extra therapeutic effectiveness with reduced toxic effects.[5] In traditional system of Indian medicine combined extract of individual plants rather than individual ones to achieve maximum theuraputic efficacy.[6]
Ethanolic extract of stem bark of Glycosmis pentaphylla, whole plant of Tridax procumbens and leaves of Mangifera indica were used in PHF. The preliminary acute toxicity of PHF and individual plant extract were not showed any significant toxic effects upto 2000 mg/kg in rodents.[7] In the light of the results of the studies on individual extracts, it was found to have good antidiabetic and antiarthritic activities. With this back ground, the present study was planned to screen antiarthritic effect of a PHF constituting ethanolic extract of stem bark of G. pentaphylla, whole plant of T. procumbens and leaves of M. indica against Freund’s complete adjuvant (FCA) induced arthritis in Female Wistar rats.
Animals
In the present investigation Wistar rats were selected to induce arthritis because rats develop a chronic swelling in multiple joints under the influence of inflammatory cells, erosion of joint cartilage due to bone destruction.[8]
Adult Wistar rats (180 g ± 10 g) of female sex were obtained from Sainath Enterprises, Hyderabad, India. The animals were housed in large, spacious polyacrylic cages at an ambient room temperature with 12-h light/12-h dark cycle with free access of food and water ad libitum. The standard pellet rat feed procured from M/s. Hindustan lever Ltd., Bangalore, India were used throughout the experimental period. The study was approved by Institutional Animal Ethical Committee of Ultra College of Pharmacy, Madurai (UCP/IAEC/2012/066 and UCP/ IAEC/2013/070). All the animals experimental procedure were carried out as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guideline.
Plant materials
Taxonomically identified leaves of M. indica, stem bark of G. pentaphylla and whole plant of T. procumbens were collected from the Alagar Kovil region, Madurai District. The collected plants were identified and authentified at the Department of Botany American College, Madurai, Tamil Nadu. The voucher specimen of the plant was deposited in the Department of Pharmacology, Ultra College of Pharmacy for further reference.
Preparation of plant extract
The air dried stem bark of G. pentaphylla, whole plant of T. procumbens and leaves of M. indica were powdered and extracted with absolute ethanol (80%) using soxlet apparatus. The extract was evaporated to dryness under reduced pressure at 60°C and stored at 4°C until use. The yield of the stem bark of G. pentaphylla, whole plant of T. procumbens and leaves of M. indica were 25.6, 25.6 and 5.34% w/w, dry weight basis respectively.
Phytochemical evaluation
Ethanolic extract of stem bark of G. pentaphylla, whole plant of T. procumbens and leaves of M. indica were reconstituted with ethanol and tested for presence of carbohydrates, proteins, sterols, alkaloids, tannins, glycosides, flavonoids, phenolic compounds and saponins.[9]
Preparation of poly herbal formulation
The poly herbal formulation was formulated by using ethanolic extracts of G. pentaphylla, T. procumbens and M. indica in the ration of 2:2:1. The poly herbal formulation (capsules) was formulated by wet granulation method using lactose 20% solution as a binder. Each 750 mg of herbal capsule contains extract of G. pentaphylla (100 mg), T. procumbens (100 mg), M. indica (50 mg) and lactose and excipients - q.s.
Standardization for formulation
Physicochemical parameters of raw materials were determined as per guidelines of World Health Organization (WHO) guideline for the quality control of herbal materials. Moisture content, total-ash value, water soluble ash, acid insoluble ash, heavy metals, water soluble extractive, alcohol soluble extractive and pH of the same were determined.[10,11]
High performance thin layer chromatography finger print analysis
Randomly few capsules were opened and the content was collected. A 20 mg sample was reconstituted with ethanol and filtered through 0.46 μm membrane. The mobile phase toluene:ethyl acetate:methanol (7:2:1) was used for chromatographic separation. A 2 μl sample (8 mm band) is spotted and the chromatogram was developed in 20 cm × 10 cm twin trough glass chamber saturated with toluene:ethyl acetate:methanol mobile phase system, and the plates were exposed for 20 min. The chromatograms were scanned by densitometer at 254 nm, 366 nm and 520 nm. The Rf values, peak areas and finger print data were recorded by winCATS 1.4.3 software (Camag Scientific Inc, United States).
Antiarthritic activity of polyherbal formulation
The male Wistar rats were divided into five different groups of six animals each as follows.
• Group I: Normal control
• Group II: Arthritic control (subcutaneous injection of FCA 0.1 ml)
• Group III: Polyherbal formulation (250 mg/kg b.w orally)
• Group IV: Polyherbal formulation (500 mg/kg b.w orally)
• Group V: Indomethacin (10 mg/kg b.w orally).
In acute toxicity studies, the polyherbal formulation did not showed any significant toxic signs upto 2000 mg/kg in rodents. Hence the present study was carried out at the dose levels of 250 and 500 mg/kg dose levels.
Prior to the experiment, paw volume (baseline) of each animal at 0 day was measured. Incomplete Freund’s adjuvant (5 mg of heat killed, powdered mycobacterium tuberculosis cell was suspended with liquid paraffin to get a 5 mg/ml suspension) was used to induce arthritis in rats. The rats were anesthetized with intraperitoneal injection of 40 mg/kg thiopentone sodium. Mineral oil was injected in right ankle joint of normal group of animals. Adjuvant arthritis was induced by subcutaneous injection of FCA (0.1 ml) into sub plantar tissue of the right hind paw of each rat. The test groups consisted of FCA injected rats challenged with the respective doses of the test drugs administered orally 24 h before FCA injection while, the vehicle control rats were injected with 0.1 ml of liquid paraffin (Incomplete Freund’s adjuvant) only. The drug treatments were continued once daily on the same time after the challenge for 20 more days. The swelling in the injected and contralateral hind paws of the rats were monitored daily using liquid displacement plethysmometer (Ugo Basile, Italy). Increase in the extent of erythema and edema of the tissues shows the severity of the inflammation. The change in body weight and paw edema were recorded at desired frequent intervals.[12,13]
At the end of the study, blood samples were withdrawn from all groups through retro-orbital plexus puncture, and whole blood was used for hematological analysis and serum was used for biochemical analysis.[14] Haematological parameters such as the hemoglobin (Hb) level, the red blood cell (RBC) count, the white blood cell (WBC) count and the erythrocyte sedimentation rate (ESR) were estimated manually using fresh blood. Serum samples were collected after centrifugation of whole blood at 3000 RPM for 20 min. Liver markers such as aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP) and creatinine were analyzed using an auto-analyzer (Vital Scientific N.V., The Netherlands). The liver enzyme levels were estimated using Lab Kit enzymatic kits. The C-reactive protein (CRP) and serum copper CRP levels estimated using the enzyme-linked immunosorbent assay kit (obtained from Alpha diagonistics Intl., USA) and the colorimetric bathocuproin disulfonate method of Zak and Landers, respectively.[15,16]
Radiographic analysis
At the end of the experiments, all rats were anesthetized with 40 mg/kg sodium thiopental intraperitoneal injection and animals were placed on X-ray plates, the projections of the left ankle joint were taken. Using tarsometatarsal region parameter such as erosion, a destruction of bony structure resulting in irregular bone surface; periosteal reaction, a fine ossified line, paralleling normal bone producing bone thickening; increase in soft tissue which was manifested as an increase in width of the soft tissue and calcification were evaluated. X-ray was taken at the knee joints for the confirmation and evaluation of the severity of arthritis in FCA induced rats.
Statistical analysis
The values are expressed as mean ± standard error of the mean. Statistical difference between normal to control and control to drug treatments were analyzed by One-way analysis of variance followed by Dunnett’s multiple comparison tests by using Graph pad prism. A P < 0.05 was considered statistically significant.
Results
Photochemical analysis
The phytochemical analysis of the ethanolic extract of G. pentaphylla showed the presence of alkaloids, tannins, flavonoids, and saponins, of T. procumbens showed the presence of alkaloids, flavonoids, saponins, and phenolic compounds, and of M. indica showed the presence of alkaloids, tannins, flavonoids, and phenolic compounds.
Preformulation studies
The study on capsules revealed that it was uniform in content and weight and further the moisture content, microbial load, limit test for heavy metals, monographic analysis of plant material, pH and disintegration time were calculated as per standard procedure and are values within the limit. Presence of heavy metals was tested by comparing the opalescence produced by the sample and standard. It was found that the test solution was less than that of standard indicates the heavy metals are present within the prescribed limits. It is shown that the formulation also complies with the WHO standards for microbial load, and hence it is safe to be taken internally.
High performance thin layer chromatography finger print analysis
Fingerprint analysis at 366 nm showed good and effective compound separation than the analysis at 254 and 520 nm. The fingerprint reports of polyherbal formulation showed presence of active compounds that present in individual herbs (data not presented). The same Rf values and peak areas were obtained in the formulation when compared with the individual Rf values and peak areas of herbal drugs. This shows that the marker compound present in individual herbals extracts and the marker compounds present in the formulation are the same.
Effect of the polyherbal formulation on body weight of arthritic rats
The changes in the body weight were monitored as apparent indicator of arthritic symptoms and the loss of body weight usually began to appear at the onset stage of arthritis. Arthritic control animals showed significant loss of body weight at the end of the study whereas PHF and indomethacin treated animals showed significant increase in body weight at the end of the study [Table 1].
| Group | Treatment | Body weight (g) | % increase in | |
|---|---|---|---|---|
| Initial | Final | body weight | ||
| I | Normal control | 210.21±1.674 228.21±4.214 | 8.5 | |
| II | Arthritic control | 208.07±3.562 196.23±3.624 | −5.76 | |
| III | PHF 250 mg/kg | 212.05±1.640 225.02±3.325 | 6.13 | |
| IV | PHF 500 mg/kg | 206.58±2.314 220.46±3.014 | 6.42 | |
| V | Indomethacin | 218.04±2.250 236.16±2.940 | 8.25 | |
PHF: Polyherbal formulation
Table 1: Percentage increase body weight compared to poly herbal formulation and indomethacin
Effect of polyherbal formulation on primary response of arthritic rat (injected paw)
One day after the FCA injection, primary arthritis of the right hind paw was induced, and inflammation was steady maintained for 21 days. After the onset of inflammation the peak incidence in swelling reached during 5–7th day with the increase in paw volume at the maximum of 1.21 ml for the arthritic control. There was a significant increase in the paw edema in all the FCA induced arthritic groups when compared to the normal control. The edema on the animals treated with PHF (250 mg/kg and 500 mg/kg) and indomethacin (10 mg/kg) group began to subside gradually (P < 0.001) when compared with arthritic control. The effect of PHF at both the selected doses on this primary reaction was found to be high at the earlier of 2nd day after FCA injection and was maintained until the termination of the experiment. The results were showed in Table 2. The results of the study shows, PHF having synergistic inhibition of paw volume on primary response.
| Treatment | Paw volume (ml)±SEM on injected paw | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | |||||||||||
| 1 | 3 | 5 | 7 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | |
| Normal control | 0.11±0.00 | 0.10±0.00 | 0.10±0.00 | 0.10±0.00 | 0.10±0.00 | 0.11±0.00 | 0.10±0.00 | 0.10±0.00 | 0.10±0.00 | 0.11±0.00 | 0.10±0.00 |
| Arthritic control | 0.78±0.00 | 0.88±0.01 | 1.07±0.00### | 1.21±0.01### | 1.10±0.00### | 0.99±0.01### | 0.81±0.01### | 0.82±0.01### | 0.84±0.01### | 0.87±0.01### | 0.92±0.01### |
| PHF 250 mg/kg | 0.79±0.01 0.81±0.01 0.84±0.01*** 0.91±0.01*** | 0.86±0.01* | 0.70±0.00*** | 0.67±0.01*** | 0.65±0.01*** | 0.61±0.01*** | 0.58±0.00*** | 0.45±0.01*** | |||
| PHF 500 mg/kg | 0.78±0.01 0.81±0.00 0.87±0.01*** 0.89±0.01*** | 0.85±0.01*** | 0.80±0.00*** | 0.64±0.00*** | 0.62±0.01*** | 0.56±0.01*** | 0.51±0.01*** | 0.40±0.01*** | |||
| Indomethacin, 10 mg/kg | 0.77±0.00 | 0.82±0.00 | 0.87±0.00*** | 0.88±0.00*** | 0.83±0.01*** | 0.79±0.00*** | 0.61±0.00*** | 0.64±0.00*** | 0.54±0.00*** | 0.51±0.01*** | 0.43±0.01*** |
Values are expressed as mean±SEM (n=6). *P<0.05; ***P<0.001compared with arthritic control, ###P<0.001 compared with normal control. Data was analyzed using one-way ANOVA followed by Dunnett’s t-test. ANOVA: Analysis of variance, SEM: Standard error of the mean, PHF: Polyherbal formulation
Table 2: Antiarthritic activity of polyherbal formulation compared with indomethacin in injected paw volume
Effect of polyherbal formulation on secondary response of arthritic rat (noninjected left paw)
The latent secondary response that occurs that after few days and characterised by joint swelling and nodule formation in the contralateral left paw was first evident on the 7th day. The administration of PHF (250 mg/kg) significantly (P < 0.001) protected against joint swelling in arthritis induced paw when compared with arthritic control group. The significant (P < 0.001) reduction was observed from day 11 to 21 in the PHF (250 mg/kg and 500 mg/kg) treated group. Throughout the 21 days experiment, there was no significant change in the paw volume of the noninflamed normal control group. The results were showed in Table 3. The results of the study shows, PHF having synergistic inhibition of paw volume on secondary response.
| Group | Treatment | Paw volume (ml)±SEM on noninjected paw | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | 7 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | ||
| II | Arthritic control | 0.51±0.00### | 0.64±0.00### | 0.81±0.01### | 0.98±0.01### | 1.10±0.01### | 1.10±0.01### | 1.11±0.01### | 1.13±0.01### | |
| I | Normal control | 0.10±0.00 | 0.11±0.00 | 0.10±0.00 | 0.10±0.00 | 0.11±0.00 | 0.10±0.00 | 0.10±0.00 | 0.10±0.00 | |
| III | PHF 250 (mg/kg) | 0.15±0.01*** | 0.24±0.01* | 0.29±0.01*** | 0.40±0.01*** | 0.44±0.01*** | 0.48±0.01*** | 0.43±0.00*** | 0.47±0.01*** | |
| IV | PHF 500 (mg/kg) | 0.15±0.01*** | 0.21±0.00*** | 0.27±0.00*** | 0.36±0.00*** | 0.41±0.01*** | 0.42±0.01*** | 0.44±0.01*** | 0.45±0.01*** | |
| V | Indomethacin, 10 mg/kg | 0.16±0.00*** | 0.20±0.00*** | 0.27±0.00*** | 0.33±0.00*** | 0.44±0.00*** | 0.42±0.00*** | 0.40±0.01*** | 0.41±0.01*** | |
Values are expressed as mean±SEM (n=6). *P<0.05; ***P<0.001 compared with arthritic control, ###P<0.001 compared with normal control. Data was analyzed using one-way ANOVA followed by Dunnett’s t-test. ANOVA: Analysis of variance, SEM: Standard error of the mean, PHF: Polyherbal formulation
Table 3: Effect of polyherbal formulation against Freund’s complete adjuvant induced paw edema on contralateral paw (secondary systemic response)
Effect of poly herbal formulation on hematological profiles of arthritic rats
The Table 4 illustrates that the arthritic rats showed a reduced RBC count, reduced Hb level and an increased ESR. All these indicate the anemic condition and are commonly noted in patients with chronic arthritis.[17] The selected doses of PHF (250 mg/kg and 500 mg/kg) treated group showed a significant (P < 0.001) increase of RBC and significant decrease (P < 0.001) in elevated ESR levels compare to arthritic control. In arthritis condition there is a mild to moderate rise in WBC count due to release of interleukin-1 beta increase the production of monocytes and colony stimulating factor.[18] The administration of PHF reverses back the elevation of WBC levels to normal level.
| Group | Treatment | Haematological parameters | |||
|---|---|---|---|---|---|
| WBC (cu.mm) | RBC (millions/cu.mm) | ESR (mm/h) | Hb (g/dl) | ||
| I | Normal control | 7010±0.06 | 6.90±0.05 | 3.54±0.10 | 14.00±0.91 |
| II | Arthritic control | 8900±0.11## | 3.02±0.23### | 7.54±0.11### | 7.36±0.210### |
| III | PHF 250 mg/kg | 7021±0.03*** | 5.21±0.24*** | 4.12±0.20*** | 12.08±0.71*** |
| IV | PHF 500 mg/kg | 7016±0.02*** | 5.91±0.11*** | 3.96±0.19*** | 13.00±0.34*** |
| V | Indomethacin, 10 mg/kg | 7012±0.01*** | 6.11±0.01*** | 3.91±0.14*** | 13.65±0.94*** |
Values are expressed as mean±SEM (n=6). ***P<0.001 compared with arthritic control. ##P<0.01; ###P<0.001 compared with normal control. Data was analyzed using one-way ANOVA followed by Dunnett’s t-test. SEM: Standard error of the mean, PHF: Polyherbal formulation, WBC: White blood cell, RBC: Red blood cell, ESR: Erythrocyte sedimentation rate, Hb: Hemoglobin
Table 4: Effect of the ethanolic extract of polyherbal formulation on hematological parameters
Effect of polyherbal formulation on biochemical parameters of arthritic rats
As a result [Table 5] of inflammation induced by adjuvant, the levels of AST, ALT and ALP were increased in all arthritis rats as compared to control rats. The PHF treated (at a doses of 250 and 500 mg/kg) animals showed significant (P < 0.001) reduction in elevated leaves of liver enzymes in arthritic rats. However, treated groups were able to reduce the AST. ALT and ALP levels better, as compared to control proving its antiarthritic efficacy. CRP is a member of the class of acute phase reactants as its levels rise dramatically during inflammatory processes. The level of CRP is significantly (P < 0.001) reduced in PHF-treated groups. Ceruloplasmin is an enzyme synthesized in the liver containing eight atoms of copper in its structure. Free copper ions are powerful catalysts of free radical damage. By binding copper, ceruloplasmin prevents free copper ions from catalyzing oxidative damage. The increased level of copper ion indicates the inflammatory condition. Serum copper concentration was measured in normal and arthritic rats.[19] The arthritic rats exhibited a significant elevation of copper level and this was significantly (P < 0.001) inhibited by PHF.
| Group | Treatment | Biochemical parameters | |||||
|---|---|---|---|---|---|---|---|
| AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Creatinine (g/dl) | Serum copper (μg/dl) | Serum CRP (µg/dl) | ||
| I | Normal control | 110.0±3.32 | 70.32±3.25 | 160.11±3.35 | 0.41 | 101.41±3.10 | 160.1±4.67 |
| II | Arthritic control | 220.21±2.52 | 136.65±2.47 | 281.62±4.12 | 1.01 | 194.24±3.24 | 370.21±4.24 |
| III | PHF 250 mg/kg | 147.6±4.24*** | 99.21±3.641*** 182.02±2.14*** | 0.45** | 129.64±4.335*** | 199.12±3.63*** | |
| IV | PHF 500 mg/kg | 131.53±3.65*** 90.35±2.014*** 172.64±3.35*** | 0.42*** | 117.32±3.643*** | 186.36±5.254*** | ||
| V | Indomethacin 10 mg/kg | 118.32±4.54*** | 84.25±3.66*** | 169.0±2.17*** | 0.44*** | 113.10±2.21*** | 181.16±3.34*** |
Values are expressed as mean±SEM (n=6). **P<0.01; ***P<0.001 compared with arthritic control. Data was analyzed using one-way ANOVA followed by Dunnett’s t-test. ANOVA: Analysis of variance, SEM: Standard error of the mean, PHF: Polyherbal formulation, AST: Aspartate amino transferase, ALT: Alanine amino transferase, ALP: Alkaline phosphatase, CRP: C-reactive protein
Table 5: Effect of polyherbal formulation on biochemical parameters
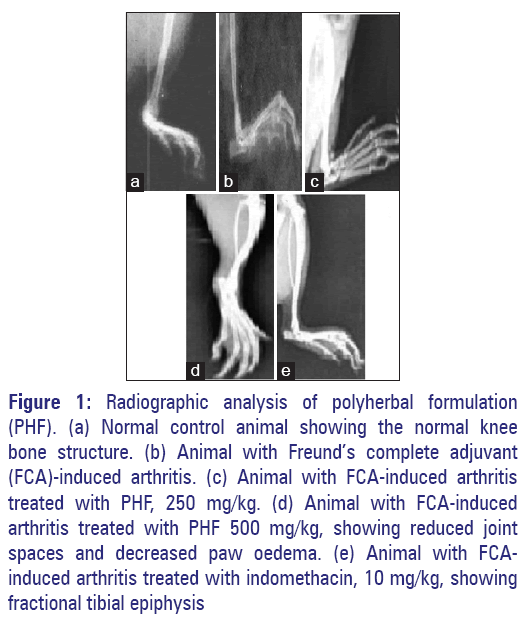
Results for radiographic analysis
The radiographic images of the knee joints of all groups of rats are shown in Figure 1. It is evident from the radiographic images that adjuvant treated rats developed periosteal reaction, irregular joint space, soft tissue swelling and joint space reduction. Whereas, in the extracts treated and in standard group the joint space appeared normal, no periosteal reaction was observed and joints appeared to be normal [Figures 1 and 2].
Figure 1: Radiographic analysis of polyherbal formulation (PHF). (a) Normal control animal showing the normal knee bone structure. (b) Animal with Freund’s complete adjuvant (FCA)-induced arthritis. (c) Animal with FCA-induced arthritis treated with PHF, 250 mg/kg. (d) Animal with FCA-induced arthritis treated with PHF 500 mg/kg, showing reduced joint spaces and decreased paw oedema. (e) Animal with FCAinduced arthritis treated with indomethacin, 10 mg/kg, showing fractional tibial epiphysis
Discussion
PHF at 250 and 500 mg/kg showed significant antiarthritic activity, and the activity was comparable with that of indomethacin. PHF significantly increased the body weight of animals compared with the arthritic controls and the antiarthritic activity of PHF is comparable with that of the standard drug indomethacin.
The PHF was formulated using the ethanolic extracts of the stem bark of G. pentaphylla, whole plant of T. procumbens, and leaves of M. Indica, which are mixed properly in 2:2:1 ratio. The antiarthritic activity of the individual plants of PHF has been proven. The stem bark of G. pentaphylla showed significant antiarthritic and antidiabetic activities against FCA induced arthritis and streptozotocin-induced diabetes, respectively, in rats at the dose levels of 400 and 800 mg/kg.[20] The aqueous extract of the leaves of M. indica showed a significant anti-inflammatory in in vivo and in vitro studies.[21,22] Ethanolic extract of the whole plant of T. procumbens showed significant antiarthritic activity against FCA induced arthritis in rats at 250 and 500 mg/kg b.wt. In some studies, the leaves of T. procumbens showed significant antidiabetic potential in rodents.[1]
Freund’s complete adjuvant induced arthritis model are extensively used to study the pathogenesis of rheumatoid arthritis for testing therapeutics and this model is characterized by a very rapid erosive disease. The bacterial peptidoglycan and muramyl dipeptide present in the FCA are responsible for the induction of adjuvant arthritis.[1,23]
In the present study, the arthritic rats exhibited a reduced RBCs count, reduced Hb level and increased ESR level. All these symptoms indicate an anemic condition, which is a common diagnostic feature in patients with chronic arthritis. The ESR is related to the number and size of the RBCs and to the relative concentration of plasma proteins, especially fibrinogen and β globulins. An increase in the ESR is an indication of active but obscure disease processes. The acute phase proteins in ESR produce inflammation similar to that produced by an injection, injury, and surgery or tissue necrosis. The treatment with extracts improved the RBCs count, Hb level and ESR to a near-normal level, indicating significant recovery from the anemic condition and arthritic progress, thus establishing that the extract has a significant role in arthritic conditions.[1]
White blood cells are a major component of the body’s immune system. Indications for a WBCs count include infections and inflammatory disease.[24] In arthritic conditions there is a mild to moderate rise in the WBC count. Apart from prostaglandin, other cyclooxygenase products and various cells involved in inflammatory changes and free radical activities have all been implicated in the development of rat adjuvant arthritis. The radiographic analysis of the knee joint in the arthritic and drug-treated animals further supported and confirmed the potent dose-dependent antiarthritic effect.[25]
The results of poly herbal formulation indicated that grater antiarthritic efficacy in poly herbal formulation than individual plant extracts.[1,5] Radiological analysis of all studies showed a marked decrease or almost completes absence of the joint spaces in the hind paw bones of arthritis induced rats.
This study is limited to preclinical examination and the polyherbal formulation is required chronic toxicity and pharmacokinetic studies to ensure the safety and efficacy of the formulation. Further, clinical trials have to be conducted to ensure and establish the safety and clinical use of formulation.
Conclusion
The polyherbal formulation exerts an antiarthritic activity by significantly altering the pathogenesis during FCA-induced arthritis in female Sprague-Dawley rats. The antiarthritic potential of the polyherbal formulation is comparable with that of indomethacin, which is evidenced by decreased levels of paw volume, AST, ALT, ALP, creatinine, serum copper and serum CRP levels.
References
- Petchi RR, Vijaya C, Parasuraman S. Anti-arthritic activity of ethanolic extract of Tridax procumbens (Linn.) in Sprague Dawley rats. Pharmacognosy Res 2013;5:113-7.
- Mukherjee PK, Venkatesh P, Ponnusankar S. Ethnopharmacology and integrative medicine - Let the history tell the future. J Ayurveda Integr Med. 2010;1:100-9.
- Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. J Clin Rheumatol 2004;10:236-45.
- Chopra A. Ayurvedic medicine and arthritis. Rheum Dis Clin North Am 2000;26:133-44.
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev 2014;8:73-80.
- Jayakumar RV. Herbal medicine for type-2 diabetes. Int J Diabetes Dev Ctries 2010;30:111-2.
- Petchi RR, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin-Nicotinamide induced diabetic Wistar rats. J Tradit Complement Med 2014;4:108-17.
- Rajendran R, Krishnakumar E. Anti-Arthritic Activity of Premna serratifolia Linn., Wood against Adjuvant Induced Arthritis. Avicenna J Med Biotechnol. 2010;2:101-6.
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytology 2011;3:10-14.
- Lohar DR, Singh R. Quality Control Manual for Ayurvedic, Siddha and Unani Medicine. Vol. 1. Ghaziabad: Department of Ayush, Ministry of Health and Family Welfare, Pharmacopoeial Laboratory for Indian Medicine; 2008. p. 21-4.
- Indian Herbal Pharmacopeia. Vol. 1. Mumbai, India: Indian Drug Manufacturer Association; 2002.
- Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact 2001;1:377-85.
- Berrington J. Biologic treatments for rheumatoid arthritis. J Orthop Nurs 2006;10:159-65.
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 2010;1:87-93.
- Landers JP. Handbook of capillary electrophoresis. Danvers, USA: CRC Press LLC; 2 nd ed. 1996. p. 567-90.
- Paval J, Kaitheri SK, Potu BK, Govindan S, Kumar RS, Narayanan SN, Moorkoth S. Comparing the anti-arthritic activities of the plants Justicia gendarussa Burm F. and Withania somnifera Linn. Int J Green Pharm 2009;3:281-4.
- Paval J, Kaitheri SK, Potu BK, Govindan S, Kumar RS, Narayanan SN, Moorkoth S. Anti-arthritic potential of the plant Justicia gendarussa Burm F. Clinics (Sao Paulo). 2009;64:357-62.
- Kshirsagar AD, Panchal PV, Harle UN, Nanda RK, Shaikh HM. Antiinflammatory and antiarthritic activity of anthraquinone derivatives in rodents. Int J Inflam. 2014;2014:690596.
- Jaijesh P, Srinivasan KK, Bhagath Kumar P, Sreejith G, Ciraj AM. Antiarthritic property of the plant Rubia cordifolia Lin. Pharmacologyonline 2008;1:107-113
- Petchi RR, Vijaya C. Anti-diabetic and anti-arthritic potential of Glycosmis pentaphylla stem bark in FCA induced arthritis and Streptozotocin induced diabetic rats. Int J Pharm Bio Sci 2012;3:328-36.
- Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res 2004;50:143-9.
- Ojewole JA. Anti-inflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem-bark aqueous extract. Methods Find Exp Clin Pharmacol 2005;27:547-54.
- Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother 1963;21:127-36.
- Sharma B, Kumar P. Extraction and pharmacological evaluation of some extracts of Tridax procumbens and Capparis deciduas. Int J Appl Res Nat Prod 2009;1:5-12.
- Kilimozhi D, Parthasarathy V, Amuthavalli N. Effect of Clerodendrum phlomidis on adjuvant induced arthritis in rats: A radiographic densitometric analysis. Int J Pharm Technol Res 2009;1:1434-41