A survey on the provision of package inserts in pediatric oral formulations by pharmaceutical manufacturers in Nigeria
- *Corresponding Author:
- Dr. Joshua Ogaji
Department of Pharmaceutics and Pharmaceutical Technology, University of Jos, PMB 2084, Jos - 930 001, Nigeria.
E-mail: ikoni.ogaji@fulbrightmail.org
Abstract
Objectives: The purpose of this study was to obtain data and understand how pharmaceuticals manufacturers care for safety and ef􀏐icacy of the products distributed for consumption in Nigeria. The study was based on a survey of wholesale pharmacies that distribute pharmaceuticals for manufacturers. The study was carried out in Jos city, a central point in the north-central Nigeria. Materials and Methods: Samples of pediatric formulations in pharmacies were obtained based on the product and the manufacturer. The packages were inspected for packing information lea􀏐let and measuring devices. In addition, the manufacturing date, expiry date, storage conditions, product registration status, and the address of the manufacturer were also noted. Simple statistic was used to analyze the data. Results: One hundred and twenty seven oral liquid formulations from 31 manufacturers were anayzed. The results showed that lea􀏐let and measuring device as well as statement on storage conditions were available in 88 (69.3%), 98 (77.2%), and 114 (89.8%), respectively, in the samples studied. All products had registration numbers, suggesting that the products were authorized for distribution; this implies that some manufacturers deliberately distribute their products in forms other than the approved form. Conclusions: Manufacturers of pharmaceutical products need to be more committed to the safety and ef􀏐icacy of their products at all times. Regulatory agency should increase surveillance to ensure that manufacturers consistently provide in their 􀏐inal packages what was declared and approved for registration.
Keywords
EfFicacy, measuring devices, medicine safety, packaging inserts, pediatric formulations
Introduction
Medicines are taken to address specific health challenges and their appropriate ingestion or usage is the key to eliciting the desired pharmacological effect.[1,2] Medicines could be described as double-edged sword, having the capacity to alleviate pain when properly administered and causing much harm when improperly used. The pharmaceutical manufacturers are responsible for the manufacture of safe, effective, and quality pharmaceutical products appropriate for the intended use. Good manufacturing practices, when carefully followed, can be of much help in achieving consistent production of safe, efficacious, and quality pharmaceutical products. Except when such a pharmaceutical product is appropriately administered, it would fail to achieve the desired effects. Many things can go wrong even after a product has been meticulously prepared according to specified standards that can rubbish the intended purpose.[3]
Patients and their representatives are usually not learned in the science of pharmacy to be able to make right judgment concerning their medications, which may be prescription or over-the-counter (OTC) medicines. Patients and their representatives must necessarily rely on the information and guidance provided by the pharmacist or the physician and sometimes by the manufacturers to enable them make appropriate and good use of the medicines in order to address their health conditions. Often, the health professionals may need supplementary information specific to the product from the manufacturer to be able to advise the patient or the representative on the product. A package insert or prescribing information is a document provided along with a prescription medication to provide useful information on the drug and the condition of use as well as what the patient may observe or look out for in the course of using the medication. Packing inserts are necessary tools to achieving dissemination of basic information on the product to a professional or a patient. The packaging inserts is a source of information to the professionals and the patient or the patients’ representative and contribute to the safe and effective use of medications. Many nations of the world recognize the role packaging insert could play in the effective and safe use of medicines and have placed demands on the manufacturers to enclose the same.[4-6] The World Health Organization[7] states that “Product information must help patients and other users to understand the medication. The patient package insert, together with the label, provides the patient with key information concerning the proper use of the product, potential adverse drug reactions and interactions, storage conditions and the expiry date. In OTC medicinal products, the package insert, together with the label, may constitute the only pharmaceutical advice that the patient receives.”
Most often, preparations for children are in liquid form or in ready to be reconstituted oral formulations and therefore require some form of measurement for the dose from the stock. Oral pharmaceutical formulations are widely used and preferred in pediatric and geriatric patients because of the difficulty in swallowing tablets or capsules by these groups of individuals. Accurate dosing of the medication, which is dependent on the device used, is very important to achieve the desired therapeutic objectives. If a dose it to be dispensed, for example, in a measurable 2.5 ml or multiple of this, it would require just that to achieve the desired effects. This is particularly important when one considers the antimicrobial agents that doses outside the optimal could lead to untoward effects of toxicity or ineffectiveness. While it is not a guarantee that the provision of accurate dosing devices would automatically lead to compliance and that other factors are also recognized, this step is the key to the process. It has been recognized that administering the right doses of oral liquid medications is crucial to achieve therapeutic goals. These doses are measured with the device that the manufacturer provided, but when this does not happen, the patient or caregiver may use any other similar, but equivalent device that would deliver doses outside that desired. Most of the household spoons that are used in administering medicines to pediatric patients have been shown not to be the same in accuracy.[8-12] It behooves the manufacturers of particularly pediatric preparations to include packaging inserts such as information leaflets and measuring devices to ensure that their medications are appropriately measured out and administered.
About 3 years ago, The National Agency for Food and Drug Administration and Control (NAFDAC; the agency saddled with the responsibility of controlling the manufacture and distribution, among other things, of pharmaceuticals and related products in Nigeria) stressed on the need for manufacturing of pharmaceutical products to enclose measuring devices for liquid preparations and information leaflets for all products. This was aimed at ensuring that the products distributed to consumers are safe and effective.
The purpose of this study was to assess the extent of inclusion or otherwise of packaging inserts and measuring devices in pediatric formulations in Nigeria. The results were analyzed and have been discussed.
Materials and Methods
The study was carried out between August 2010 and February 2011 and involved visiting registered pharmaceutical premises in Jos metropolis. Jos is central to many commercial cities in Nigeria, serving either as a depot to service northern states or is within 3 hours drive from the major commercial cities in the north, namely Kaduna and Kano, as well as to Federal Capital Territory, Abuja. Jos is a suitable city for this kind of study because of its unique location in the middle belt of Nigeria and the presence or access of pharmaceutical manufacturers from where many other northern states are serviced.
The major pharmaceutical distributors were visited, and samples of common remedies such as anti-malarials, antibiotics, analgesic, and anti-inflammatory, as well as anti-tussive were obtained from the shelves at random according to the number of companies manufacturing the product. If there was no package insert, more packs from the manufacturer were opened to rule out the chances that it was an omission in the one sampled. The information that was looked for included identifiable address of the manufacturer, the presence, or otherwise of information leaflet, inclusion or otherwise of measuring device, wherever applicable.
Results
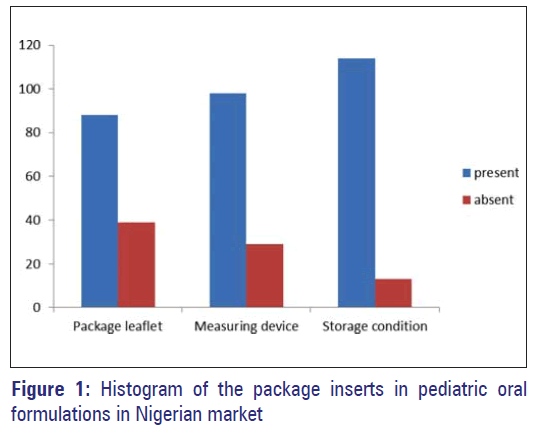
A total of 127 oral liquid formulations from 31 manufacturers were sampled in the survey. Of the 31 companies, 4 were public liability companies, while the rest were limited liability companies. Nigeria has over a hundred registered pharmaceutical companies engaging in secondary or tertiary manufacture of pharmaceuticals of different dosage forms. The packaged pediatric formulations that were provided with leaflet, measuring device, and stated storage conditions were respectively 88 (69.3%), 98 (77.2%), and 114 (89.8%) as shown in Figure 1.
A total of 127 oral liquid formulations from 31 manufacturers were sampled in the survey. Of the 31 companies, 4 were public liability companies, while the rest were limited liability companies. Nigeria has over a hundred registered pharmaceutical companies engaging in secondary or tertiary manufacture of pharmaceuticals of different dosage forms. The packaged pediatric formulations that were provided with leaflet, measuring device, and stated storage conditions were respectively 88 (69.3%), 98 (77.2%), and 114 (89.8%) as shown in Figure 1.
Table 1 shows the summary on packaging information insert, measuring devices, and statement on the storage conditions of the formulation as provided by the manufacturers. Eighty six percent (86%) of the anti-microbial agents group of pediatric formulations had packaging information leaflet and the corresponding value for anti-malarial agent was 76%. The order of decreasing provision of packaging information leaflet was anti-microbial> anti-malarial> analgesic = anti-tussive. The Table also shows the data on the provision of measuring devices by the manufacturers. All the anti-malarial agents sampled had measuring devices in their packages. On the other hand, the analgesic, anti-tussive, and anti-microbial preparations with measuring devices were, respectively, 90, 82, and 56% of the number sampled.
| Class of drug | Packaging Insert | Measuring device | Direction on storage | ||||
|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | Present | Absent | ||
| Anti-malarials (n = 25) | 19 (76) | 6 (24) | 25 (100) | 0 (0) | 25 (100) | 0 (0) | |
| Anti-microbial (n = 50) | 43 (86) | 7 (14) | 28 (56) | 22 (44) | 43 (86) | 7 (14) | |
| Analgesic (n = 30) | 15 (50) | 15 (50) | 27 (90) | 3 (10) | 24 (80) | 6 (20) | |
| Anti-tussive (n = 22) | 11 (50) | 11 | (50) | 18 (81.8) | 4 (18.2) | 22 (100) | 0 (0) |
Figures in parentheses are given in percentage
Table 1: Data generated from the survey on the inclusion or otherwise of packing insert and measuring devices in pediatric oral liquid preparation packages by pharmaceutical manufactures.
Discussion
The high level of inclusion of packaging information inserts is an indication of the awareness of the manufacturers of the importance of the supplementary information to proper use, safety, and effectiveness of their products. In Nigeria, the National Agency for Food and Drug Administration and Control is the body charged with the responsibility of registering pharmaceutical products intended to be advertised or sold for human and animal use. Such products are registered with a unique number against set protocols which include the provision of appropriate packaging information insert among others. The presence of this number in all the products sampled indicated that manufacturers had once substantially complied with the regulatory requirements for them to be licensed to manufacture, advertise, distribute and sell the pediatric formulations. It is unlikely that the manufacturers of the products without the packing information leaflets were unaware of the need to include them, indicating a deliberate attempt to undermine the safety of their products. Pharmaceutical manufacturers must be self-motivated and go beyond merely meeting regulatory requirements at product registration point to ensure that they do all that is possible to assure safety and effectiveness of their products. The importance of information to effective and safe use of medicines cannot be overemphasized and relevant packaging information plays a key role. It may be argued that the manufacturer do provide information on the outer (folding box or the secondary package) and sometimes on the label. The secondary packaging is usually limited in space to contain all the drug related information that may be required. The present study identified missing gaps in the information provided on the folding boxes that would have been complemented in the package information leaflet. None of the defaulting manufacturer was of the public liability company category, and the defaulting companies spread across the country from their addresses. It is not clear why many manufacturers failed to include the measuring device with their products, particularly the antimicrobial formulations. It has been observed that many of the household spoons do not have the same accuracy. It would be a great risk to allow the patient to use whatever measuring device they have to take their anti-microbial medication. Such an approach would lead to medication errors and raise safety and efficacy concerns. It is obvious that duly packaged products were presented to the regulatory agency at the time of registration of the products and it would be a breach of trust to present something different to the public. On the other hand, the regulatory agencies should continually carry out post marketing surveillance and apply the appropriate actions on the defaulting pharmaceutical manufacturers so as to assure safety of the products to the users.
Conclusion
A survey of level of inclusion of packaging inserts such as the package information leaflet and measuring devices of pediatric formulations marketed in Nigeria was carried out. Products from public and limited liability companies were sampled. Manufacturers of pharmaceuticals in Nigeria are aware of the importance of the packaging inserts in the safe and efficacious use of their medicines; few of them are yet to apply this knowledge to the benefit of the users of their products. Manufacturers should be deliberate in their intention to contribute to the wellbeing of the consumers of their products rather than merely meet the regulatory requirements. On the other hand, the regulatory agency should intensify their post-marketing surveillance to ensure that manufacturers consistently adhere to the rule of the game.
Source of Support: Nil, Conflfl ict of Interest: Nil
References
- Brodie DC, Parish PA, Poston JW. Societial needs for drugs and drugs related services. Am J Pharm Educ 1980;44:276-8.
- Webb DG, Davies JG, McRobbie D. Clinical pharmacy process. In: Walker R, Whittlesea C, editors. Clinical Pharmacy and Therapeutics.
- 5th ed. London: Churchill Livingstone; 2012. p. 3-13.
- Woolf SH, Kuzel AJ, Dovery SM, Phillips RL Jr. A string of mistakes: The importance of cascade analysis in describing, counting, and preventing medical errors. Ann Fam Med 2004;2:317-26.
- Fuchs J, Hippius M, Schaefer M. A survey of package inserts use by patients. Hosp J Pharm Eur 2005;21:29.
- Fuchs J, Hippius M, Schaefer M. Analysis of German package inserts. Int J Clin Pharmacol Ther 2006;44:8-13.
- Fuchs J, Banow S, Gorbert N, Hippius M. Importance of package information in the European Union. Medicinal and pharmaceutical experts questioning results. Pharm Ind 2007;69:165-72.
- WHO. Guidelines on Packaging for Pharmaceutical Products, 2002; Technical Report series No. 902, Annex 9.
- Bayor MT, Kipo SL, Ofori-Kwarye K. The accuracy and quality of household spoons and enclosed dosing devices used in the administration of oral liquid medications in Ghana. Int J Pharm Pharm Sci 2010;2:150-3.
- Hyam E, Brawer M, Herman J, Zvieli S. Whats in a Teaspoon Under dosing with acetaminophen in family practice. Fam Pract 1989;6:221-3.
- John JM. Preventing medication errors at home. J Pharm Pract 2005;18:141-4.
- Jonville AP, Autret E, Bavoux F, Bertrand PP, Barbier P, Gauchez AS. Characteristics of medication errors in pediatrics. Ann Pharmacother 1991;25:1113-8.
- Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract 2000;49:741-4.