A clinical study on drug-related problems associated with intravenous drug administration
- *Corresponding Author:
Abstract
Objective: To evaluate prevalence, types, and severity of potential adverse drug‑drug interaction in medicine out‑patient department. Materials and Methods: A single‑point, prospective, and observational study was carried out in medicine OPD. Study began after obtaining approval Institutional Ethics Committee. Data were collected and potential drug‑drug interactions (pDDIs) were identified using medscape drug interaction checker and were analyzed. Result: A total of 350 prescriptions with mean age 52.45 ± 14.49 years were collected over a period of 5 months. A total of 2066 pDDIs were recorded with mean of 5.90 ± 6.0. The prevalence of pDDI was 83.42%. Aspirin was most frequently prescribed drug in 185 (10.15%) out of total of 1821 drugs It was also the most frequent drug implicated in pDDI i.e. in 48.16%. The most common pDDI identified was metoprolol with aspirin in 126 (6.09%). Mechanism of interactions was pharmacokinetic in 553 (26.76%), pharmacodynamic in 1424 (68.92%) and 89 (4.30%) having an unknown mechanism. Out of all interactions, 76 (3.67%) were serious, 1516 (73.37%) significant, and 474 (22.94%) were minor interaction. Age of the patients (r = 0.327, P = 0.0001) and number of drugs prescribed (r = 0.714, P = 0.0001) are significantly correlated with drug interactions. Conclusion: Aspirin being the most common drug interacting. The use of electronic decision support tools, continuing education and vigilance on the part of prescribers toward drug selection may decrease the problem of pDDIs.
Keywords
Drug-related problems, error, intravenous
Introduction
Intravenous (IV) therapy is complex, potentially dangerous and error prone, thus the need for strategies to reduce the risk and complications. [1] Infusion therapy through IV access is a therapeutic option used in the treatment of many hospitalized patients. [2] Infusion medications are associated with high risk of harm. Once injected, reversal is almost impossible unless an antidote exists. [3] The IV route of medication administration has many advantages and benefits. The most important are the immediate therapeutic effect of medications. It can sustain high plasma drug levels and may be used when a person has difficulty in swallowing. [4] The drug when given intravenously will reach the target rapidly. [5] Thus, IV route is the preferred route when the patient is critically ill. However, there are also a lot of possible direct and negative side effects such as pulmonary complications, thrombophlebitis, and infection with the possibility of sepsis. [4] There have been reports of death and harm following medication errors such as wrong dose drug diluents and cross contamination errors. Thus, the primary focus should be to identify IV therapy associated drug-related problems (DRPs). [3]
Drug-therapy problems in intravenous administration
Drug-therapy (related) problem can be defined as an event or circumstance involving drug treatment that actually or potentially interferes with the patient experiencing an optimum outcome of medical care. [6] DRPs can originate when prescribing, dispensing or administering medications. It may lead to substantial morbidity and mortality as well as increase the health care expenditure, thus affecting both patients and the society. [7]
Wrong diluents
In the German and French hospitals, the most frequent error was preparing the medicine with the wrong diluents. The use of the wrong diluents may cause a reduction in the solubility of the medicine powder being reconstituted that can lead to powder particulates being administered to the patient. The use of the wrong diluents can also lead to a reduction in the stability and activity of medicine and possible drug precipitation. [8]
Incompatibilities
Intravenous access is usually limited and often need to have medications administered simultaneously through the same line. This is facilitated by a y-site connector where the medications mix in the lumen of the tubing for up to 1 min prior to being infused into the patient. Not all medications can be mixed together as all are not compatible with each other. [9] Incompatibility is an undesirable reaction that occurs between the drug and the solution, container or another drug. Administering incompatible medications together through the same line can result in negative consequences and even death in some extreme cases. [10] The three incompatibilities associated with IV administration are physical, chemical and therapeutic incompatibility. [11]
Wrong rate and wrong time errors
It was reported that at UK hospitals, the most frequent IV medication errors were related to the administration rate, usually higher than that recommended. The administered drug characteristics, fast rates of drug administration are associated with pain, phlebitis, and other complications. [8]
Complications of intravenous therapy
Intravenous therapy presents a potential risk to patient safety with associated risks varying from minor complications to death. As more number of patients are becoming acutely ill, the numbers of patients requiring IV therapies are increasing. Maintaining the patient’s vascular access throughout treatment is difficult because a number of complications including phlebitis, infiltration, extravasations, and infections may occur. [12] Complications increase hospital stays, duration of therapy, and can also put the patients at risk of other medical problems. [13]
Pharmacist role in intravenous administration
The mission of the profession of pharmacy is to improve public health through ensuring safe, effective, and appropriate use of medications. [14] Clinical Pharmacist can play a significant role in nurse training as an effective method to reduce the rate of errors in the hospital. One obvious solution to aid in the process of DRPs could be considering pharmacy services in IV product preparation by implementing protocol prepared by Clinical Pharmacist and establishment of reporting error systems. [15]
Pharmacist role to provide expert advice on compatibility and stability for the use of multiple drugs if required for IV administration, update staff on new clinical practice guidelines and help to interpret guidelines as they apply to patients with advanced illness. Thus, permanent supervision and involvement of Clinical Pharmacist is important. [16]
Materials and Methods
A prospective observational study was carried out over the duration of 4 months from April, 2013 to July, 2013 at Private Corporate Hospital, Coimbatore, India and the study was approved by Institutional Ethics Committee. The patients who received more than two IV medications irrespective of their age and gender were enrolled in our study. Patients from Intensive Care Unit (ICU) and Oncology Department were excluded from the study.
Definition, assessment, and description of intravenous drug-related problems
Intravenous DRP was defined as an error of using wrong rate or dilution in the context of administering medications intravenously. We addressed DRPs as wrong rate, wrong dilution procedure, incompatibility complications developed after IV administration. The subjects in this study were classified based on their diagnosis into various departments (Neurology, Cardiology, Endocrinology, Nephrology, Ortho etc.). DRPs were further categorized based on type of IV administration (IV bolus, continuous IV infusion).
Data collection
For each patient, basic demographic characteristics as well as occurrence and descriptive factors of each IV DRPs were documented into a structured case record form. DRPs documented include incompatibilities, rate of administration errors, dilution errors, and complications developed. Incompatibilities were categorized into actual and observed. Actual incompatibilities were referred to those incompatibilities documented on a theoretical basis from the medication chart, whereas observed incompatibilities were referred to those incompatibilities that were seen in patients. Confidentiality of the entire patient’s data was maintained.
Statistical analyses
Drug-related problems and its impact on gender and venous access site, since patients were treated with central and peripheral line were investigated. Data were analyzed by using SPSS software version 14.0.1 manufactured by SPSS Inc., Chicago, IL.
Results
A total of 110 patients were involved in this study during the period of 4 months. The male (69.09%) population was predominant when compared with the female population [Table 1]. Majority of the male were seen in the age group of 60-69 (15.45%) years, whereas female were seen mainly in the age group of 40-49 (9.09%) years.
| Variables | Males | Females | Relative risk | 95% CI | P value |
|---|---|---|---|---|---|
| Central line | |||||
| No error | 5 | 3 | 1.455 | 0.823-2.568 | 0.1337 |
| Error | 10 | 1 | |||
| Drug related problems | |||||
| Yes | 41 | 11 | 1.307 | 1.016-1.681 | 0.0360* |
| No | 35 | 23 | |||
| Infusion rate error | |||||
| Yes | 8 | 4 | 0.9608 | 0.6305-1.464 | 0.8473 |
| No | 68 | 30 | |||
| Error in dilution | |||||
| Yes | 8 | 1 | 1.320 | 1.010-1.726 | 0.1798 |
| No | 68 | 33 | |||
| Incompatibility | |||||
| Yes | 37 | 8 | 1.370 | 1.070-1.740 | 0.0130* |
| No | 39 | 26 | |||
| Complications | |||||
| Yes | 13 | 1 | 1.410 | 1.153-1.730 | 0.0390* |
| No | 63 | 33 | |||
*P<0.05, CI: Confidence interval
Table 1: Gender wise association of variables in study population
The DRPs seen in our study population receiving IV medications were incompatibilities, complications, rate of administration error and dilution errors. Among the 110 patients, nearly half of the patients (46.3%, n = 51) were reported with DRPs. Patients receiving IV medications through peripheral line (82.72%, n = 91) was predominant than those receiving central lines (17.27%, n = 19). Out of 80 DRPs (72.72%), 61 problems (55.4%) were seen in patients given IV medications through peripheral line, whereas 19 (17.27%) DRPs were seen in patients given medications through the central line [Table 2].
| Drug-related problems | Central line | Peripheral line | Relative risk | 95% CI | P value |
|---|---|---|---|---|---|
| Complications | |||||
| Yes | 4 | 10 | 1.829 | 0.7072-4.728 | 0.2312 |
| No | 15 | 81 | |||
| Error in dilution | |||||
| Yes | 1 | 8 | 0.6235 | 0.0937-4.148 | 0.6098 |
| No | 18 | 83 | |||
| Infusion rate error | |||||
| Yes | 3 | 9 | 1.531 | 0.5211-4.500 | 0.4531 |
| No | 16 | 82 | |||
| Incompatibilities | |||||
| Yes | 11 | 34 | 1.986 | 0.8679-4.545 | 0.0978 |
| No | 8 | 57 | |||
CI: Confidence interval
Table 2: Intravenous access site association with variables in study population
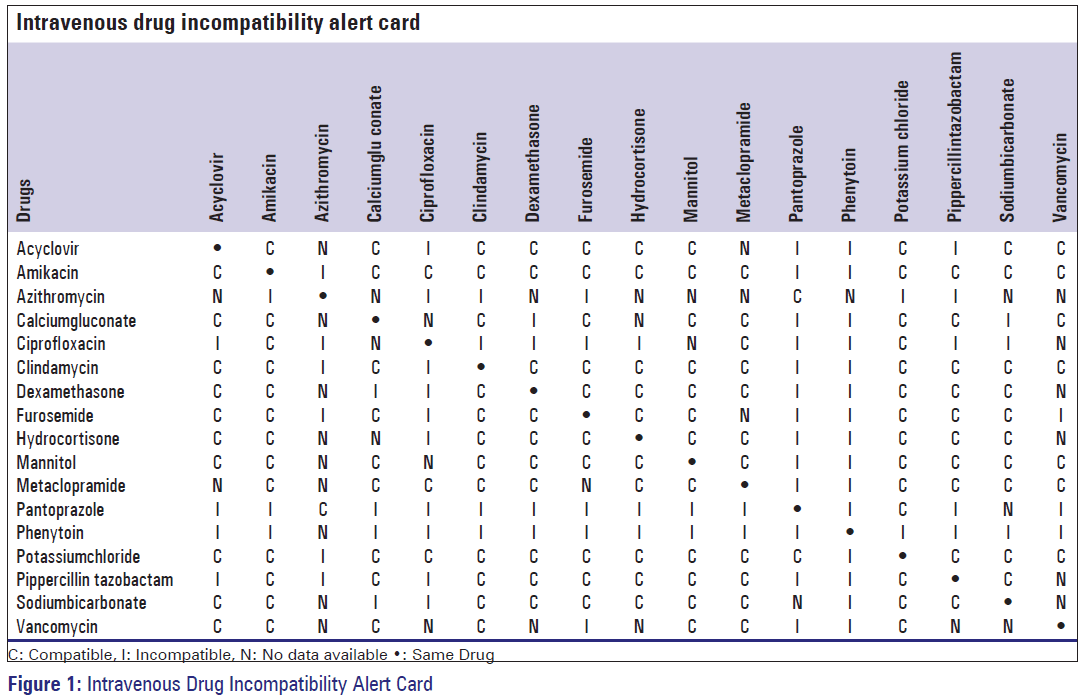
Among the DRPs majority were incompatibilities (40.9%, n = 45), followed by complications developed (12.7%, n = 14) after IV administrations, errors in the rate of administration were accounted for 12 patients (10.9%) and errors in the dilution accounted for nine patients (8%). The incompatibilities documented were categorized into observed and actual incompatibilities. Among the 45 incompatibilities documented, 11.8% (n = 13) of the incompatibilities were observed [Figure 1] and 29% (n = 32) were actual incompatibilities. From the observed incompatibilities, the most common reason for the cause of incompatibility was the development of precipitate (10.9%, n = 12). Only one incompatibility was attributed to color change over time. The most common drugs involved in incompatibilities were Pantoprazole, Phenytoin, Mannitol and Pipercillin. Based on our observation and results, IV drug compatibility-alert card was prepared in order to enhance the rational use of IV medication and patient safety [Figure 2].
The most common IV incompatibilities were reported from Neurology Department (10.9%, n = 12), out of which three were observed and nine were actual incompatibilities. It was followed by Cardiology Department (7.2%, n = 8), Endocrinology (5.4%, n = 6), Nephrology. Of 45 incompatibilities, majority of the incompatibilities were seen between one bolus and an infusion (57.7%, n = 26), incompatibilities between two IV bolus drugs were seen in 35.55% of the incompatibilities (n = 16) and only three incompatibilities involved two infusion drugs.
Discussion
This study was carried out to determine the DRPs involved in IV medication administration and develop strategies to reduce and prevent the occurrence of DRPs during administration of IV medications. Such strategies will improve the quality of preparation and administration of IV medications and reduce the DRPs in the long run.
The predominance of patients receiving more than two IV medications were male (69.09%, n = 76) and female receiving more than two medications were only 30.90% (n = 34). Our study results are more similar to the study conducted by Ponni et al., results. [17]
Studies have reported that IV administration of drugs has a higher risk and severity of errors than any other medication administration. [18] The DRPs seen in our study population receiving IV medications were incompatibilities, rate of administration errors, dilution errors, and complications. Incompatibilities were dominant than all other DRPs. Among the 110 patients, nearly half of the patients (46.3%, n = 51) were reported with DRPs. When compared to other European studies, [18] it was observed that our study results indicated less number of DRPs.
In a randomized control trial, [19] majority patients received IV medications through peripheral line, but the DRPs were seen mainly in patients with IV medications through the central line. Whereas in our study, patients receiving IV medications through peripheral line (82.72%, n = 91) was predominant than those receiving central lines (17.27%, n = 19). Out of the 80 DRPs (72.72%) seen, 61 problems (55.4%) were seen in patients given IV medications through peripheral line, whereas 19 (17.27%) DRPs were seen in patients given medications via central line. Since, the study was carried out only at general and specialty wards, not in ICU.
Direct observational studies performed in the United Kingdom and Germany revealed overall error rates of 49% and 48%, respectively. [18] Whereas among the DRPs in our study majority seen were incompatibilities (40.9%, n = 45), followed by complications developed (12.7%, n = 14) after IV administrations, errors in the rate of administration were accounted for 12 patients (10.9%) and errors in the dilution accounted for nine patients (8%). In contrast, another study revealed that wrong rate of administration was the most frequent error, followed by omissions and wrong dose. [20]
Administering incompatible medications together through the same line can result in negative consequences or death in extreme cases. [9] The large number of incompatibilities seen in our study may be due to lack of knowledge regarding drug incompatibility and their consequences for the patient.
Among the 45 incompatibilities documented, 11.8% (n = 13) of the incompatibilities were observed and 29% (n = 32) were actual incompatibilities. From the observed incompatibilities, the most common reason for the cause of incompatibility was the development of precipitate (10.9%, n = 12). Only one incompatibility was attributed to color change over time. The most common drugs involved in incompatibilities were pantoprazole, phenytoin, mannitol, and piperacillin. The study conducted by Kanji et al., were matched with our study results. [9]
Results from different studies are difficult to compare because of differing methods of analysis. Further to study the significance of DRPs among gender, statistical analysis was performed. Our results revealed that the relative risk for all DRPs were >1. It indicates that there is a large difference between the groups compared. Furthermore, significant association was observed between total DRPs and gender (P = 0.03). Similarly, when comparing DRPs individually significant association was observed in case of incompatibilities (P = 0.013) and complications (P = 0.039). Increased complications seen in men possibly are due to the high number of incompatibilities in men.
Even though, there was a difference between central and peripheral line, we have performed statistical analysis to know the risk of individual DRPs among patients with central and peripheral line. It reveals that there was a significant difference in cases of infusion rate error, complications and incompatibilities. However, no significant association was seen in patients receiving IV medications through central and peripheral line (P > 0.05).
A European study reported that effective strategies are needed to reduce the harmful errors during IV drug administration. [21] Based on our observation and results, IV drug compatibility-alert card was prepared in order to enhance the rational use of IV medication and patient safety.
Limitations
The study has certain limitations. Since it was a pilot study, it was carried out in wards and did not include ICU. Longer period of data collection from ICU, will definitely be associated with other IV administration related DRPs. The time of administration of certain IV medications was different from the time of data collection. Such data were collected from patient records and verbally from nurses. Further studies may be carried out in a large sample size to predict more DRPs.
Conclusion
Although the majority of the DRPs do not cause significant harmful clinical outcomes to patients, training needs as well as plans should be proposed to reduce such complexity. Among the DRPs, simultaneous IV administration of two incompatible drugs was the main predicament faced. As the outcome from the study, an IV drug compatibility-alert card was prepared and distributed to the wards to help and minimize any confusion regarding the commonly used IV drugs. It is recommended that check list should be introduced in wards to encourage monitoring dilution and administration rate of IV infusions. Thus, permanent supervision and involvement of Clinical Pharmacist will improve the quality of preparation and administration of IV medications and will also reduce the DRPs.
Acknowledgments
The authors are gratefully thankful to Dr. Nalla G. Palaniswami, Chairman and Managing Director of Kovai Medical Center and Hospital, Coimbatore and Dr. Thavamani D. Palaniswami, Trustee, Kovai Medical Center Research Cancer and Educational Trust, Coimbatore for providing necessary facilities and continuous encouragement.
Source of Support
Nil
Conflict of Interest
None declared.
References
- McDowell SE, Mt-Isa S, Ashby D, Ferner RE. Where errors occur in the preparation and administration of intravenous medicines: A systematic review and Bayesian analysis. Qual Saf Health Care 2010;19:341-5.
- Summa-Sorgini C, Fernandes V, Lubchansky S, Mehta S, Hallett D, Bailie T, et al. Errors associated with IV infusions in critical care. Can J Hosp Pharm 2012;65:19-26.
- Cousins DH, Upton DR. Medication error 79: How to prevent IV medicine errors. Pharm Pract 1997;7:310-1.
- Bernaerts K, Evers G, Sermeus W. Frequency of intravenous medication administration to hospitalised patients: Secondary data-analysis of the Belgian nursing minimum data set. Int J Nurs Stud 2000;37:101-10.
- Ong WM, Subasyini S. Medication errors in intravenous drug preparation and administration. Med J Malaysia 2013;68:52-7.
- Mil FV. Drug related problems: A cornerstone for pharmaceutical care. J Malta Pharm Pract 2005;10:5-8.
- Ruths S, Viktil KK, Blix HS. Classification of drug-related problems. Tidsskr Nor Laegeforen 2007;127:3073-6.
- Cousins DH, Sabatier B, Begue D, Schmitt C, Hoppe-Tichy T.Medication errors in intravenous drug preparation and administration: A multicentre audit in the UK, Germany and France. Qual Saf Health Care 2005;14:190-5.
- Kanji S, Goddard R, Kanji S, Goddard R, Donnelly R, McIntyre, Turgeon A, Coons P, et al. Physical Compatibility of Drug Infusions used in Canadian Intensive Care Units: A Program of Research. Canadian Patient safety Institute 2010.
- Newton DW. Drug incompatibility chemistry. Am J Health Syst Pharm. 2009; 66 (4): 348-357.
- Cayo L. Compatibility of Commonly Used Intravenous Drugs. In: Pharmacy Practice News. 2010: 41-46.
- Ingram P, Lavery I. Peripheral intravenous therapy: Key risks and implications for practice. Nurs Stand 2005;19:55-64.
- Complications of peripheral iv therapy. NMIE. 2008; 6 (1): 14-18.
- Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: Roles, responsibilities, and functions of pharmacists and pharmacy technicians. J Am Pharm Assoc (2003) 2010;50:e35-69.
- Majid AK. The roles of clinical pharmacy in reducing medication errors. Int Res J Pharm 2012;3:76-83.
- Abbasinazari M, Zareh-Toranposhti S, Hassani A, Sistanizad M, Azizian H, Panahi Y. The effect of information provision on reduction of errors in intravenous drug preparation and administration by nurses in ICU and surgical wards. Acta Med Iran 2012;50:771-7.
- Ponni J, Acthyuth KY, Mohanta GP, Kabalimurthy J. Studies on the use of intravenous fluid management in the first week of post-operative period of gastrointestinal surgery. Indian J Pharm Pract 2012;5:16-20.
- Westbrook JI, Rob MI, Woods A, Parry D. Errors in the administration of intravenous medications in hospital and the role of correct procedures and nurse experience. BMJ Qual Saf 2011;20:1027-34.
- Wilson D, Verklan MT, Kennedy KA. Randomized trial of percutaneous central venous lines versus peripheral intravenous lines. J Perinatol 2007;27:92-6.
- Wirtz V, Taxis K, Barber ND. An observational study of intravenous medication errors in the United Kingdom and in Germany. Pharm World Sci 2003;25:104-11.
- Taxis K, Barber N. Causes of intravenous medication errors: An ethnographic study. Qual Saf Health Care 2003;12:343-7.