Effects of Rosuvastatin Alone or in Combination with Omega-3 Fatty Acid on Adiponectin Levels and Cardiometabolic Profile
- *Corresponding Author:
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Adiponectin is an important adipocyte related protein that has been postulated to participate in prevention of the development of metabolic syndrome. The relationship between adiponectin serum levels and risk of coronary artery disease (CAD) has been widely investigated and remains controversial. The aim of the present study was to evaluate the effects of rosuvastatin and/or omega 3 fatty acid on adiponectin serum levels in patients with insulin resistance (IR) and CAD. Patients and Methods: This study involved 87 patients with CADs and IR of different etiology, the patients were divided into three groups; 24 patients on treatment with rosuvastatin, 22 patients on treatment with omega 3 fatty acid, 23 patients on treatment with omega 3 fatty acid and rosuvastatin, 18 patients were not previously or currently treated with either rosuvastatin or omega 3 fatty acid, those regarded as control patients. Anthropometric measures, adiponectin serum levels, and other biochemical parameters were assessed in each treated group. Results: Rosuvastatin therapy leads to a significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 6.76 ± 1.03 ng/mL compared to control P < 0.01. Omega 3 fatty acid therapy leads to a significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 6.11 ± 1.29 ng/mL compared to control P < 0.01. Rosuvastatin plus omega 3 fatty acid therapy lead to a significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 7.99 ± 1.76 ng/mL compared to control P < 0.01. Conclusions: Rosuvastatin and/or omega 3 fatty acid lead to significant cardiometabolic protection through an increment in adiponectin serum levels.
Keywords
Adiponectin, omega-3 fatty acid, rosuvastatin
Introduction
Coronary artery disease (CAD) is one of the most common causes of mortality and morbidity worldwide, caused by coronary atherosclerosis that mediated by dyslipidemia and inflammatory processes; CAD presented as stable angina or acute coronary syndrome that includes acute myocardial infarction and unstable angina. [1] The relationship between adiponectin serum levels and risk of CAD has been widely investigated and remains controversial. [2]
Adiponectin is an adipocytokine secreted from adiposities; it possesses significant insulin-sensitizing, anti-inflammatory, and antiatherogenic effects. [3]
Adiponectin accounts for 0.01% of plasma protein with a half-life of 2.5 h; normal adiponectin plasma level is 5–10 μg/mL with higher levels in female than males due to sexual dimorphism. [4] Adiponectin plasma forms are of two types, high-molecular weight (biological active form) and low-molecular weight. In addition, high-molecular weight adiponectin levels are positively associated with CAD and negatively associated with risk of type 2 diabetes mellitus (DM), but this is not true to the low-molecular weight adiponectin. [5] Adiponectin serum levels are inversely correlated with body mass index (BMI), visceral obesity, and insulin resistance (IR); thus, it regarded as an indicator and predictor of noninsulin dependent DM, insulin resistant, and overt hyperglycemia. Since adiponectin is responsible for provoking of fatty acid oxidation and skeletal muscles glucose uptake, inhibition of liver gluconeogenesis, and stimulation of pancreatic β-cells for insulin secretion, thereby adiponectin plays an important role in linking glucose metabolism with visceral adiposity. [6] Reduction of adiponectin levels in obesity predisposes for the development of CAD due to the reduction of adiponectin-mediated antioxidative, anti-inflammatory, antiapoptotic, and antiatherogenic effects. [7] High adiponectin levels considered as protective against the development of CAD in younger populations, but there is controversy regarding its cardioprotective role in elderly. [8]
IR is a failure of cells to respond the normal insulin action leading to high insulin levels and hyperglycemia, it may be uncertain and undetected until the development of overt diabetes commonly progressing into metabolic syndrome. [9] In IR, there are downregulations of adiponectin and adiponectin receptors leading to a reduction of insulin sensitivity and augmentations of vascular endothelial dysfunctions. [10]
Adiponectin and adiponectin receptors may be a target for different drugs and herbs, berberine, and other herbs such as gingerol, curcumin, and capsaicin have been shown to augment and stimulate adiponectin, which per se explain the potential antidiabetic effects of berberine. [11,12] Furthermore, studies with animal model demonstrated that mice that are fed with the docosahexaenoic acid and omega-3 fatty acid have been shown to stimulate adiponectin gene expression. [13]
Many previous studies have been revealed that statins are valuable in increasing the adiponectin levels and reduction of cardiovascular complications, but lipophilic statins such as atorvastatin were failed in improving the optimal high-molecular weight adiponectin while hydrophilic statins such as rosuvastatin increase the high-molecular weight adiponectin. [14]
The aim of the present study was to evaluate the effects of rosuvastatin and/or omega-3 fatty acid on adiponectin serum levels in patients with IR and CAD.
Patients and Methods
This study was conducted at the Department of Clinical Pharmacology and Therapeutic in conjunction with the Department of Internal Medicine, College of Medicine, Al-Mustansiriya University from June to November 2015, Baghdad, Iraq. This study was permitted and approved by the Ethical and Scientific Committee; all enrolled patients gave informed written consent for initiation of the study (study permission no. 229/A2).
This was a cross-sectional study involved 87 patients with CADs and IR of different etiology. The patients were recruited from the Coronary Care Unit at Al-Yarmouk Teaching Hospital. The patients were included using the New York Heart Association classification, [15] and categorized according to the treatment history into three groups; Group (A): 24 patients on treatment with rosuvastatin 20 mg/day, Group (B): 22 patients on treatment with omega-3 fatty acid 400 mg/day (180 mg EPA + 120 DHA), Group (C): 23 patients on treatment with omega-3 fatty acid and rosuvastatin, Group (D): 18 patients were neither previously nor currently treated with either rosuvastatin or omega-3 fatty acid were regarded as control patients.
An exclusion criterion included patients with chronic liver disease, chronic renal failure, sepsis, stroke, and rheumatic and connective tissue diseases.
Biochemical measurements
after an overnight fasting, 10 ml of venous blood was drained from antecubital site, 7 ml put into planar tubes for routine investigations, and 3 ml put into ethylenediaminetetraacetic acid tubes and centrifuged at 2000 r/min for insulin, adiponectin, and cardiac troponin-I (cTnI) assessments. cTnI serum levels were estimated by ELISA kit method catalog (kit number E-ELRI1253 pg/mL, Elbascience, China), adiponectin serum levels were assessed through ELISA kit method (Cat. No. AG-45A-0001EK-KI01 ng/mL, Incheon, Korea), insulin serum levels were measured by ELISA kit method (ACS-180 mU/L, Ciba Corning Diagnostics, Medified, USA). Total cholesterol, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-c) were estimated by specific enzymatic colorimetric kits while low-density lipoprotein cholesterol (LDL-c) was determined by Friedewald et al. method; [16] then from lipid profile, different measures could be estimated through specific equations. Atherogenic index = log (TG/HDL), [17] atherogenic coefficient (AC) = total cholesterol-HDL/HDL, [18] very LDL (VLDL) = TG/5, and cardiac risk ratio CCR = total cholesterol/HDL. [19] Fasting and postprandial blood glucose were determined by ELISA kit method (Glucose Colorimetric Assay kit, K686-100, BIOVISION, China).
Anthropometric measurements
weight and height were measured through stadiometer and BMI with formula; BMI = body weight (kg)/height (m2). [20]
Waist circumference was estimated in the standing position at midpoint between the upper border of the iliac crest and lower border of the last rib through graduated tape in centimeters. [21]
Hip circumference was estimated in the standing position, the largest distance between the greater trochanters, [22] also measured waist–hip ratio and a waist height ratio.
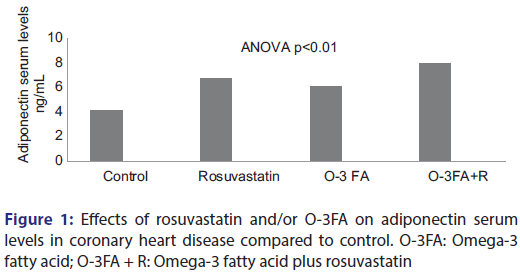
Basal adiposity index (BAI) is calculated by the following formula: [23]
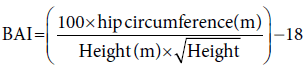
Lean body mass (LBM) was calculated according to the Boer formula as LBM = (0.32810 × body weight) + (0.33929 × height) − 29.5336, [24] and body fat % = (1.2 × BMI) + (0.23 × age) − (10.8 × gender) − 5.4, (1 for male and 0 for female). [25]
Cardiometabolic measurements
Blood pressure measurements of enrolled patients were assessed by standard mercury sphygmomanometer after rest at sitting position at the right arm. A resting twelfth leads electrocardiography (ECG) was assessed through digital ECG portable device recorder (GE Marquette Mac 800 Ecg-EkgMachine, USA).
Criteria for IR include, fasting serum insulin >25 μIU/L and postprandial blood glucose PPG 140–197 mg/dl.
The homeostatic model assessment-IR (HOMA-IR) was measured by specific equation that depends on fasting blood glucose (mg/dl) and insulin (μIU/L), the HOMA-β cell function. [26]
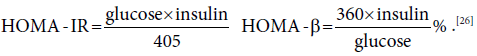
Insulin sensitivity was assessed by Quantitative Insulin Sensitivity Check Index (QUICKI),
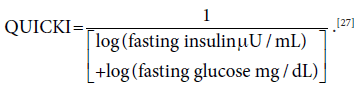
Statistical analysis
Data were presented as mean ± standard deviation and variables were compared by ANOVA test which determined the significance among groups followed by post hoc test, considering P < 0.05 statistically significant. Data were statistically analyzed through statistical package for social sciences software version 19.0 (SPSS 19.0, 2010, IBM Corp., NY, USA).
Results
A total number of 97 patients were recruited in this study, ten patients were withdrawn due to personal reasons. Thus, 87 patients continued in this study. They were 24 (27.58%) patients treated with rosuvastatin, 22 (25.28%) patients on treatment with omega-3 fatty acid, 23 (26.43%) patients on treatment with omega-3 fatty acid and rosuvastatin, and 18 (20.68%) patients treated with neither of rosuvastatin nor omega-3 fatty acid. Majority of the patients were well compliant with treatment, with 57:30 male:female ratios, respectively. The patients in this study were associated with disease condition hyperlipidemia (94.25%), hypertension (90.80%), and IR (51.72%). The main duration of CAD was 4.55 ± 1.49 years with 64.36% of them having a positive family history for CAD. The patient characteristics are presented in Table 1.
| Patient characteristics | Mean±SD, n (%) |
|---|---|
| Number | 87 |
| Age | 48.38±11.73 |
| Gender | |
| Male | 57 (65.51) |
| Female | 30 (34.48) |
| Hyperlipidemia | 82 (94.25) |
| Hypertension | 79 (90.80) |
| Insulin resistance | 45 (51.72) |
| CAD | 87 (100) |
| Stable angina | 17 (19.54) |
| Unstable angina | 12 (13.79) |
| STEMI | 44 (50.57) |
| NSTEMI | 14 (16.09) |
| Types of MI | |
| Anterior | 12 (13.79) |
| Posterior | 11 (12.64) |
| Anterioseptal | 13 (14.94) |
| Inferior | 17 (19.54) |
| Anteriolateral | 5 (5.74) |
| Onset of chest pain | 3.77±1.23 |
| Duration of chest pain | 8.71±1.62 |
| Troponin-I | |
| Positive | 70 (80.45) |
| Negative | 17 (19.54) |
| CAD management | |
| Aspirin | 67 (77.011) |
| Clopidogrel | 55 (63.21) |
| Enoxaparin | 86 (98.85) |
| Rosuvastatin | 24 (27.58) |
| Omega-3 fatty acid | 22 (25.28) |
| Rosuvastatin + Omega-3 fatty acid | 23 (26.43) |
| Metoprolol | 45 (51.72) |
| ACEI | 22 (25.28) |
| Calcium channel blockers | 10 (11.49) |
| Morphine | 79 (90.80) |
| DC shock | 2 (2.29) |
| Duration of rosuvastatin therapy (month) | 9.77±2.12 |
| Duration of omega-3 fatty acid therapy | 4.83±1.83 |
| Duration of combination therapy | 5.29±1.55 |
| Rosuvastatin off therapy | 18 (20.68) |
| Family history of CAD | |
| Positive | 56 (64.36) |
| Negative | 31 (35.63) |
Results are expressed as mean ± SD, n (%). CAD: Coronary artery disease, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, ACEI: Angiotensin converting enzyme inhibitor, MI: Myocardial infarction
Table 1: Baseline patient characteristics
There were no significant differences in anthropometric profiles between rosuvastatin-treated patients, omega-3 fatty acid-treated patients, rosuvastatin plus omega-3 fatty acid-treated patients, and control (P > 0.05) [Table 2].
| Variables | Control (n=18) | Rosuvastatin(n=24) | Omega-3 fattyacid (n=22) | Rosuvastatin andomega-3 fattyacid (n=23) | F statistic | P | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Age (year) | 42.43±13.72 | 43.87±12.61 | 43.55±11.67 | 42.87±10.33 | 0.061 | 0.9802 | 8.4163-10.2238 | |
| Height (cm) | ||||||||
| Male | 178.44±16.82 | 177.39±16.55 | 177.49±15.55 | 176.39±15.58 | 0.0553 | 0.9828 | 12.4698-13.3209 | |
| Female | 165.66±13.73 | 166.84±12.94 | 166.81±13.88 | 166.75±13.99 | 0.0335 | 0.9917 | 9.9583-10.5135 | |
| Weight (kg) | 93.63±19.43 | 97.55±17.29 | 95.73±16.44 | 97.55±16.29 | 0.2329 | 0.8732 | 10.2133-13.2263 | |
| BMI (kg/m2) | 31.76±8.62 | 33.02±8.22 | 32.41±9.66 | 33.04±6.72 | 0.1063 | 0.9562 | −5.5564-6.3590 | |
| WC (cm) | ||||||||
| Male | 101.66±11.62 | 101.67±11.83 | 100.68±12.69 | 102.67±12.84 | 0.0984 | 0.9607 | −10.0317-8.3973 | |
| Female | 90.42±10.53 | 91.88±10.62 | 92.66±10.75 | 91.81±11.29 | 0.144 | 0.9332 | −7.3822-8.2048 | |
| HC (cm) | ||||||||
| Male | 102.29±14.93 | 102.33±11.69 | 103.62±13.78 | 103.71±16.82 | 0.0644 | 0.9785 | −11.7093-12.3753 | |
| Female | 104.83±16.43 | 103.77±16.77 | 105.75±15.23 | 104.99±15.79 | 0.0596 | 0.9808 | −14.1910-13.5083 | |
| WH r | ||||||||
| Male | 0.99±0.09 | 0.99±0.08 | 0.97±0.04 | 0.98±0.06 | 0.4132 | 0.7439 | −0.0566-0.0430 | |
| Female | 0.86±0.061 | 0.88±0.063 | 0.87±0.07 | 0.87±0.054 | 0.3578 | 0.7836 | −0.0309-0.0376 | |
| W-Htr | ||||||||
| Male | 0.56±0.09 | 0.57±0.04 | 0.57±0.09 | 0.57±0.08 | 0.0814 | 0.97 | −0.0525-0.0585 | |
| Female | 0.54±0.06 | 0.55±0.02 | 0.55±0.07 | 0.55±0.05 | 0.173 | 0.9144 | −0.0329-0.0401 | |
| BAI | ||||||||
| Male | 24.95±5.88 | 25.32±5.97 | 25.47±6.33 | 25.74±6.44 | 0.0574 | 0.9818 | −4.6753-5.1416 | |
| Female | 31.07±9.62 | 30.16±9.05 | 31.09±9.33 | 30.63±8.77 | 0.4757 | 0.7001 | −15.2244-13.8659 | |
| Body fat (%) | ||||||||
| Male | 19.8±3.88 | 20.0±5.75 | 19.3±3.61 | 20.7±3.69 | 0.3946 | 0.7572 | −3.3776-4.0480 | |
| Female | 30.9±8.79 | 30.7±8.99 | 32.1±8.99 | 31.3±7.85 | 0.113 | 0.9523 | −7.2800-7.2256 | |
| Lean body mass | ||||||||
| Male | 66.6±11.72 | 67.9±12.55 | 67.1±11.72 | 67.9±10.77 | 0.0611 | 0.9801 | −8.2783-8.9637 | |
| Female | 53.7±9.33 | 55.2±9.97 | 54.7±9.44 | 55.2±8.33 | 0.1135 | 0.9519 | −6.0950-7.1077 | |
Results are expressed as mean ± SD. BMI: Body mass index, WC: Waist circumference, HC: Hip circumference, WH r: Waist-hip ratio,
W-Ht r: Waist height ratio, BAI: Body adiposity index, CI: Confidence interval, SD: Standard deviation
Table 2: The differences in anthropometric profiles between rosuvastatin-treated, omega-3 fatty acid-treated and rosuvastatin-omega 3 fatty acid-treated patients compared with control
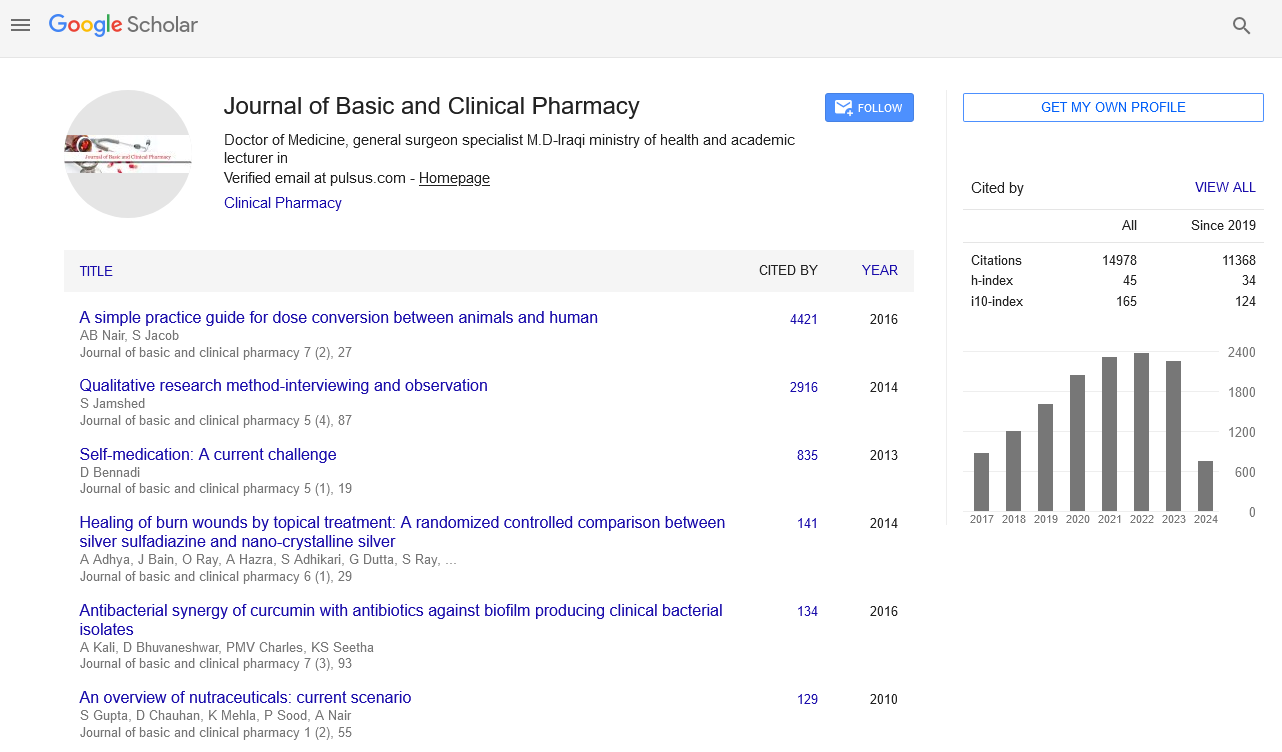
Rosuvastatin therapy leads to a significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 6.76 ± 1.03 ng/mL compared to control P < 0.0001. Similarly, rosuvastatin significantly improved IR, fasting blood glucose, and HOMA-IR compared to control P < 0.05. Rosuvastatin also significantly reduced the levels of total cholesterol, total TG, VLDL, LDL-c, atherogenic index, and AC (P < 0.0001) and showed a significant elevation in HDL-c (P < 0.05). Moreover, rosuvastatin reduced systolic and diastolic blood pressures (DBPs) with a significant reduction in cardiac risk ratio compared to control (P < 0.05) [Table 3].
| Variables | Control (n=18) | Rosuvastatin(n=24) | Omega-3 fattyacid (n=22) | Rosuvastatin and omega-3 fatty acid (n=23) | F statistic | P | 95% CI |
|---|---|---|---|---|---|---|---|
| Adiponectin (ng/mL) | 4.1±0.99 | 6.76±1.03 | 6.11±1.29 | 7.99±1.76 | 30.371 | 0.0000* | 1.5826-2.2382 |
| Cardiac troponin-I (pg/mL) | 75.33±13.29 | 73.67±12.72 | 74.88±12.48 | 73.22±11.83 | 0.1312 | 0.9413 | −11.9201-9.1517 |
| Insulin (μIU/L) | 12.9±2.82 | 10.75±2.93 | 9.62±3.11 | 9.88±1.82 | 5.8307 | 0.0012* | −4.3638-1.2017 |
| FBG (mg/dL) | 109.66±12.65 | 100.63±6.83 | 90.54±7.63 | 97.64±10.52 | 13.8292 | 0.000* | −16.7755-4.2584 |
| PPG (mg/dL) | 144.73±12.83 | 143.99±14.69 | 136.83±13.73 | 137.83±14.83 | 1.7895 | 0.1555 | −12.2870-4.6460 |
| HOMA-IR | 3.5±1.09 | 2.7±0.87 | 2.2±0.94 | 2.38±1.01 | 6.7561 | 0.0004* | −1.5956-0.4245 |
| HOMA-β | 99.52±9.81 | 102.84±12.66 | 125.75±11.59 | 102.67±12.66 | 22.5646 | 0.0000* | −6.3734-8.9013 |
| QUICKI | 0.32±0.055 | 0.33±0.042 | 0.34±0.045 | 0.34±0.011 | 1.1016 | 0.3533 | −0.0232-0.0411 |
| Total cholesterol (mg/dL) | 321.77±22.91 | 199.65±12.71 | 244.63±11.62 | 164.88±15.79 | 363.287 | 0.0000* | −122.120-57.277 |
| Total triglycerides (mg/dL) | 221.73±13.85 | 164.63±13.62 | 141.69±22.52 | 133.82±13.89 | 113.15 | 0.0000* | −70.536-18.235 |
| HDL-c (mg/dL) | 44.76±7.89 | 51.66±8.94 | 46.73±6.86 | 53.83±9.61 | 5.2191 | 0.0024* | −0.0022-8.6292 |
| VLDL (mg/dL) | 44.34±8.83 | 32.92±6.33 | 28.33±4.72 | 26.76±5.99 | 28.9588 | 0.0000* | −16.7319--1.1890 |
| LDL-c (mg/dL) | 232.66±22.62 | 115.06±12.89 | 183.62±14.22 | 84.26±6.85 | 429.616 | 0.0000* | −129.5714-79.8924 |
| Atherogenic index | 0.335±0.044 | 0.143±0.023 | 0.122±0.031 | 0.035±0.0036 | 396.157 | 0.0000* | −0.2150-0.0865 |
| Atherogenic coefficient | 6.19±1.33 | 2.86±0.87 | 4.23±1.77 | 2.06±0.99 | 40.3314 | 0.0000* | −4.3720-0.1751 |
| CCR | 4.95±1.55 | 3.86±0.95 | 5.23±1.88 | 3.06±0.97 | 11.6826 | 0.0000* | −2.2126-0.2506 |
| SBP (mmHg) | 155.73±21.62 | 136.79±22.61 | 150.72±14.62 | 141.86±11.94 | 4.6652 | 0.0046* | −4.1058-18.9523 |
| DBP (mmHg) | 91.72±11.86 | 83.22±11.82 | 87.52±8.65 | 82.69±9.63 | 3.2324 | 0.0264** | −17.1144-7.5316 |
Results are expressed as mean±SD, *P<0.05, **P<0.01. FBG: Fasting blood glucose, PPG: Postprandial glucose, HOMA-IR: Homeostatic model assessment insulin resistance, HOMA-β: Homeostatic model assessment β cell function, QUICKI: Quantitative Insulin Sensitivity Check Index, HDL-c: High-density lipoprotein-cholesterol, LDL-c: Low-density lipoprotein-cholesterol, VLDL: Very low-density lipoprotein, CCR: Cardiac risk ratio, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, SD: Standard deviation, CI: Confidence interval
Table 3: Variations in adiponectin serum levels, biochemical, and cardiometabolic profiles in three different treated groups compared to the control
Omega-3 fatty acid therapy leads to a significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 6.11 ± 1.29 ng/mL compared to control (P < 0.0001), also omega-3 fatty acid significantly improved IR, fasting blood glucose, HOMA-β, and HOMA-IR compared to control (P < 0.01). Regarding the effect of omega-3 fatty acid on lipid profiles, it reduced the total cholesterol level, total TG, VLDL, LDL-c, atherogenic index, and AC significantly (P < 0.01) with no significant elevation in HDL-c compared to control [Table 3].
Rosuvastatin plus omega-3 fatty acid therapy lead to significant elevation in adiponectin serum levels from 4.1 ± 0.99 ng/mL to 7.99 ± 1.76 ng/mL compared to control (P < 0.01); rosuvastatin plus omega-3 fatty acid also improved IR, fasting blood glucose, HOMA-IR, and improved lipid profile significantly P < 0.01. Moreover, rosuvastatin plus omega-3 fatty acid also reduced the systolic and DBPs P < 0.05 with a significant reduction in cardiac risk ratio compared to control (P < 0.01). All treated groups with rosuvastatin and/or omega-3 fatty acid showed no significant effects on cTnI [Table 3].
There was significant differences in adiponectin serum levels between treated groups and nontreated group (control), this difference was highly significant between omega-3 fatty acid and rosuvastatin plus omega-3 fatty acid (P = 0.00001), whereas less significant between rosuvastatin and rosuvastatin plus omega-3 fatty acid-treated group and insignificant between rosuvastatin and omega-3 fatty acid-treated group [Table 4].
| Biochemical parameters | Post-hoc test (Tukey HSD) (P) | ANOVA test | ||||||
|---|---|---|---|---|---|---|---|---|
| C versus R | C versus O | C versus OR | R versus O | R versus OR | O versus OR | F | P | |
| Adiponectin (ng/mL) | 0.0000* | 0.0000* | 0.0000* | 0.3455 | 0.0104‡ | 0.0000* | 30.37 | 0.0000* |
| Cardiac troponin-I (pg/mL) | 0.0602 | 0.0015* | 0.0036* | 0.4944 | 0.6899 | 0.9884 | 5.830 | 0.0012* |
| Insulin (μIU/L) | 0.0156‡ | 0.0000* | 0.0007* | 0.0029* | 0.7017 | 0.0652 | 13.82 | 0.0000* |
| FBG (mg/dL) | 0.9983 | 0.3000 | 0.4114 | 0.3213 | 0.4454 | 0.9953 | 1.7895 | 0.1555 |
| PPG (mg/dL) | 0.0482‡ | 0.0004* | 0.0025* | 0.3095 | 0.6741 | 0.9253 | 6.756 | 0.0004* |
| HOMA-IR | 0.8059 | 0.0000* | 0.8332 | 0.0000* | 0.9683 | 0.0000* | 22.56 | 0.0000* |
| HOMA-β | 0.8593 | 0.4140 | 0.4051 | 0.8385 | 0.8338 | 0.8355 | 1.1016 | 0.3533 |
| QUICKI | 0.0820 | 0.0000* | 0.8862 | 0.0000* | 0.0000* | 0.0000* | 363.28 | 0.0000* |
| Total | 0.0000* | 0.0000* | 0.0000* | 0.0001* | 0.0000* | 0.3811 | 113.15 | 0.0000* |
| Triglycerides (mg/dL) | 0.0501 | 0.8831 | 0.0054* | 0.2045 | 0.8148 | 0.03‡ | 5.2191 | 0.0024* |
| HDL-c (mg/dL) | 0.0000* | 0.0000* | 0.0000* | 0.0863 | 0.0089* | 0.8495 | 28.95 | 0.0000* |
| VLDL (mg/dL) | 0.1859 | 0.0000* | 0.9111 | 0.0000* | 0.0000* | 0.0051* | 429.61 | 0.0000* |
| LDL-c (mg/dL) | 0.0010* | 0.0302‡ | 0.9316 | 0.0623 | 0.0000* | 0.0000* | 396.15 | 0.0000* |
| Atherogenic index | 0.0000* | 0.0000* | 0.0000* | 0.0026* | 0.1458 | 0.0000* | 40.33 | 0.0000* |
| Atherogenic coefficient | 0.0603 | 0.9182 | 0.0002* | 0.0060* | 0.1976 | 0.0000* | 11.68 | 0.0000* |
| SBP (mmHg) | 0.0066* | 0.8209 | 0.0795 | 0.0527 | 0.7738 | 0.3636 | 4.66 | 0.0046* |
| DBP (mmHg) | 0.0545 | 0.5945 | 0.0387‡ | 0.5138 | 0.9982 | 0.4203 | 3.232 | 0.0264‡ |
Significance of differences among treated groups presented via ANOVA test and Tukey HSD. *P<0.01, ‡P<0.05; C versus R: Control versus rosuvastatin, C versus O: Control versus omega-3 fatty acid, C versus OR: Control versus rosuvastatin + omega-3 fatty acid, R versus O: Rosuvastatin versus omega-3 fatty acid, R versus OR: Rosuvastatin versus rosuvastatin + omega-3 fatty acid, O versus OR: Omega-3 fatty acid versus rosuvastatin + omega-3 fatty acid. FBG: Fasting blood glucose, PPG: Postprandial glucose, HOMA-IR: Homeostatic model assessment insulin resistance, HOMA-β: Homeostatic model assessment β cell function, QUICKI: Quantitative Insulin Sensitivity Check Index, HDL-c: High-density lipoprotein cholesterol, LDL-c: Low-density lipoprotein cholesterol, VLDL: Very low-density lipoprotein, HSD: Honestly significant difference, SBP: Systolic blood pressure, DBP: Diastolic blood pressure
Table 4: Variations in adiponectin serum levels, biochemical, and cardio-metabolic profiles in treated groups compared to the control
Rosuvastatin plus omega-3 fatty acid therapy revealed more significant elevation in adiponectin serum levels compared to control and other treated groups [Figure 1].
Adiponectin serum levels in patients with CAD that were not treated with either rosuvastatin or omega-3 fatty acid showed significant negative correlation with HOMA-β, QUICKI, total TG, VLDL, CCR, and DBP. Omega-3 fatty acid-treated patients’ adiponectin serum levels were negatively correlated with insulin levels, fasting blood glucose, postprandial glucose, HOMA-β, total cholesterol, total TG, VLDL, LDL-c, atherogenic index, AC, CCR, diastolic and systolic blood pressure (SBP), whereas showed significant positive correlation with HDL-c. The same results were observed in rosuvastatin-treated patients except this correlation was insignificant with VLDL, atherogenic index, and SBP. Finally, adiponectin serum levels in rosuvastatin plus omega-3 fatty acid-treated patients had significant negative correlations with insulin levels, total cholesterol, total TG, VLDL, LDL-c, CCR, SBP, and DBP, and significant positive correlation with QUICKI and HDL-c [Table 5].
| Cardiometabolic Variables | Control (n=18) | Omega-3 FA (n=22) | Rosuvastatin (n=24) | Omega-3 FA + rosuvastatin (n=23) | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Insulin (μIU/L) | −0.3321 | 0.062 | −0.9616 | <0.0001* | −0.3896 | 0.017‡ | −0.4364 | 0.01‡ |
| Cardiac troponin-I (pg/mL) | −0.0576 | 0.336 | −0.1493 | 0.19 | −0.1992 | 0.123 | −0.0707 | 0.293 |
| FBG (mg/dL) | −0.1524 | 0.213 | −0.6049 | 0.001* | −0.9945 | <0.0001* | −0.107 | 0.239 |
| PPG (mg/dL) | −0.1753 | 0.188 | −0.4862 | 0.006* | −0.4017 | 0.014‡ | −0.2567 | 0.08 |
| HOMA-IR | −0.1945 | 0.168 | 0.0863 | 0.439 | −0.3693 | 0.022‡ | −0.2075 | 0.121 |
| HOMA-β | −0.7894 | <0.0001* | −0.9814 | <0.0001* | −0.4268 | 0.01‡ | −0.2184 | 0.111 |
| QUICKI | −0.4193 | 0.027‡ | 0.2498 | 0.187 | −0.3693 | 0.022‡ | 0.9509 | <0.0001* |
| Total cholesterol (mg/dL) | −0.1918 | 0.171 | −0.4699 | 0.007* | −0.6202 | <0.0001* | −0.5858 | 0.001* |
| Total triglycerides (mg/dL) | −0.777 | <0.0001* | −0.6779 | <0.0001* | −0.9458 | <0.0001* | −0.5564 | 0.001* |
| HDL-c (mg/dL) | 0.233 | 0.236 | 0.5666 | 0.005* | 0.9652 | <0.0001* | 0.956 | <0.0001* |
| VLDL (mg/dL) | −0.9791 | <0.0001* | −0.521 | 0.003* | 0.3621 | 0.066 | −0.9509 | <0.0001* |
| LDL-c (mg/dL) | −0.2741 | 0.099 | −0.3242 | 0.046‡ | −0.9896 | <0.0001* | −0.3858 | 0.02‡ |
| Atherogenic index | −0.1596 | 0.205 | −0.6286 | <0.0001* | −0.1448 | 0.183 | −0.2075 | 0.121 |
| Atherogenic coefficient | −0.0908 | 0.29 | −0.3592 | 0.031‡ | −0.6531 | <0.0001* | −0.018 | 0.379 |
| CCR | −0.9926 | <0.0001* | −0.8892 | <0.0001* | −0.3693 | 0.022‡ | −0.9782 | <0.0001* |
| SBP (mmHg) | −0.1652 | 0.199 | −0.4155 | 0.016‡ | −0.286 | 0.057 | −0.8222 | <0.0001* |
| DBP (mmHg) | −0.995 | <0.0001* | −0.6145 | <0.0001* | −0.9973 | <0.0001* | −0.6656 | <0.0001* |
P value was calculated at 95% CI; *P<0.01, ‡P<0.05, FBG: Fasting blood glucose, PPG: Postprandial glucose, HOMA-IR: Homeostatic model assessment insulin resistance, HOMA-β: Homeostatic model assessment β cell function, QUICKI: Quantitative Insulin Sensitivity Check Index, HDL-c: High-density lipoprotein cholesterol, LDL-c: Low-density lipoprotein cholesterol, VLDL: Very low-density lipoprotein, CCR: Cardiac risk ratio, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, CI: Confidence interval, FA: Fatty acid
Table 5: Correlation of adiponectin levels with cardiometabolic profiles
Discussion
Adiponectin is known for an important physiological effects such as anti-inflammatory, vascular protection, antidiabetic, and cardioprotective effects. [28] Thus, hypoadiponectinemia may predispose patients to the cardiovascular complications and augment the risk of type 2 DM [29] which corresponds with results of the present study. Our study demonstrated a low adiponectin serum levels in patients with CAD and IR.
In acute CAD, the role of protective and beneficial effects of adiponectin remained obscure and uncertain due to various cellular and molecular mechanisms. Earlier studies by Sattar et al. failed to reveal cardiometabolic protection afforded by adiponectin in CAD, [30] therefore, many clinical studies were conducted by stimulating adiponectin effects using drugs or recombinant adiponectin to exert cardioprotection. [31]
The present study showed significant effect of rosuvastatin in elevation of adiponectin serum level with amelioration of cardiometabolic risk factors of CAD patients.
Animal model study reported by Shibata et al., 2005 demonstrated that statin therapy leads to increase adiponectin serum levels and improvement of HOMA-IR in patients with acute coronary syndrome and IR through inhibition of tumor necrosis factor-induced myocardial ischemia and upregulation of myocardial adiponectin gene that regulates cardiac fatty acid oxidation and glucose uptake. [32] In addition, oxidative stress during myocardial infarction leads to myocardial IR and cardiac dysfunction, thus rosuvastatin acts as a direct antioxidant or indirectly through stimulation of adiponectin which is a potent antioxidant. [33,34]
Moreover, rosuvastatin in therapeutic doses in the present study decreased serum insulin levels and improved HOMA-IR. However, study by Thongtang et al., revealed that the highest rosuvastatin dose leads to a moderate increment in the insulin levels. [35]
Rosuvastatin therapy also leads to significant amelioration in lipid profile, atherogenic index, AC, cardiac risk ratio, and reduction in blood pressure compared to patients not taking rosuvastatin; these findings correspond with many previous studies that revealed significant lipid lowering effects of rosuvastatin with preservation of endothelial function through stimulation of vascular endothelial nitric oxide. [36] Rosuvastatin such as pitavastatin is hydrophilic, which exert potent cardiometabolic protection, glycemic control, and adiponectin activation more than lipophilic statins, [37] which further explains the cardioprotective and glucometabolic effects of rosuvastatin in the present study.
On the other hand, the patients with CAD treated with omega-3 fatty acid showed insignificant differences in the anthropometric parameters compared to the control, this finding was similar to study by Rafraf et al. which pointed out toward insignificant effects of omega-3 fatty acid therapy on anthropometrics profile. [38] Therapy with omega-3 fatty acid in patients with CAD led to significant increases in adiponectin serum levels compared to control with significant improvement in HOMA-IR, HOMA-β, insulin levels, and fasting blood glucose, these findings were inconsistent with Yamamoto et al. results which revealed that administration of omega-3 fatty acid produced significant anti-inflammatory effects in patients with CAD associated with hyperlipidemia through an increase in adiponectin serum levels. [39] Omega-3 fatty acid increases adiponectin serum levels through activation of adipocytes peroxisome proliferator-activated receptor gamma that augments adiponectin synthesis and secretion. [40] Lorente-Cebrián et al. in 2006 disclosed an increased in the adiponectin by omega-3 fatty acid lead to reduction of fasting insulin levels and attenuation of IR due to adiponectin-insulin-sensitizing property. [41]
Indeed, omega-3 fatty therapy in the present study led to a significant amelioration of lipid profile, atherogenic index, and AC with insignificant elevation in HDL-c levels while the effect on the cardiac risk ratio and blood pressures were insignificant, these observations were consistent with the finding of Dokholyan et al. study that displayed insignificant effects of omega-3 fatty therapy on the reduction of blood pressure in hypertensive patients. [42]
Furthermore, dual effects of omega-3 fatty acid plus rosuvastatin led to more pronounced effects on cardiometabolic risk profile through significant increases in adiponectin levels, amelioration of lipid profiles, cardiac risk ratio, atherogenic index, AC, reduction in blood pressure, and improvement of IR. As previously reported, combinations of rosuvastatin and omega-3 fatty acid therapy were associated with a higher reduction of cardiovascular risk scores compared to the either rosuvastatin or omega-3 fatty acid monotherapy. [43]
Moreover, Mindrescu et al. study observed that omega-3 fatty acid supplementation in rosuvastatin-treated patients lead to significant endothelial function and improvement in the vasoactive effect of rosuvastatin. Deficiency in free fatty acids upregulates HMG-CoA reductase activity, which leads to rosuvastatin resistance, subsequently downregulation of nitric oxide, endothelial dysfunction followed by IR. [44] Hence, combined therapy of omega-3 fatty acid and rosuvastatin lead to more cardiometabolic protection as presented in our study through reduction in the blood pressure and cardiac risk ratio.
Mente et al. observed that the variation in adiponectin serum levels was linked to the ethnicity, especially South Asian and Caucasian peoples. IR in these people was correlated low adiponectin levels, whereas this correlation was not observed with Indian peoples. [45] This observation corresponds with the findings of the current study since all enrolled patients were Iraqi patients.
The present study demonstrated a protective role of adiponectin against acute coronary heart disease (CHD) contrasting with the findings of earlier study by Dekker et al. which showed an association between high adiponectin levels and high cardiovascular mortality risk. [46] This elevation might due to use of common medication that used in CHD such as statins, fibrate, and angiotensin converting enzyme inhibitors which generally increase serum adiponectin levels, or due to the elevation of brain natriuretic levels in patients with heart failure as natriuretic levels stimulate adiponectin secretion. [47]
Finally, adiponectin serum levels were positively correlated with plasma HDL-c and QUICKI and negatively correlated with other measured parameters consistent with the finding of Li et al. [48]
Limitation of the present study
The study had small sample size which might lead to over or under estimations of adiponectin serum levels. Gender differences in adiponectin serum levels were not evaluated since it is documented as higher in women, [49] we measured only total adiponectin levels not high-molecular weight adiponectin (which is more active and linked to insulin sensitivity). We also did not include hemoglobin A1c and brain natriuretic peptides in our estimations. Moreover, physical activity, dietary habit, and other adipocytokines were also not evaluated in this cross-sectional study. Our study also limited to only one type of ethnic population.
Conclusion
Rosuvastatin and/or omega-3 fatty acid lead to significant cardiometabolic protection through an increment in adiponectin serum levels in patients with CAD.
Acknowledgment
The authors would like to thank all staff in the Department of Clinical Pharmacology, College of Medicine for their support and cooperation.
Financial support and sponsorship
Department of Clinical Pharmacology, College of Medicine.
Conflicts of interest
There are no conflicts of interest.
References
- Wal P, Wal A, Nair VR, Rai AK, Pandey U. Management of coronary artery disease in a tertiary care hospital. J Basic Clin Pharm 2013;4:31-5.
- Shams M, Rasekhi Kazerouni A, Ostovan MA, Omrani GR. The relationship between serum adiponectin levels with the presence and severity of coronary artery disease. Arch Iran Med 2012;15:611-6.
- Ikeda Y, Hama S, Kajimoto K, Okuno T, Tsuchiya H, Kogure K. Quantitative comparison of adipocytokine gene expression during adipocyte maturation in non-obese and obese rats. Biol Pharm Bull 2011;34:865-70.
- Coppola A, Marfella R, Coppola L, Tagliamonte E, Fontana D, Liguori E, et al. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int J Cardiol 2009;134:414-6.
- Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab 2007;9:282-9.
- Meyvis K, Verrijken A, Wouters K, Van Gaal L. Plasma adiponectin level is inversely correlated with albuminuria in overweight and obese nondiabetic individuals. Metabolism 2013;62:1570-6.
- Bueno AC, Sun K, Martins CS, Elias Junior J, Miranda W, Tao C, et al. A novel ADIPOQ mutation (p.M40K) impairs assembly of high-molecular-weight adiponectin and is associated with early-onset obesity and metabolic syndrome. J Clin Endocrinol Metab 2014;99:E683-93.
- Rizza S, Gigli F, Galli A, Micchelini B, Lauro D, Lauro R, et al. Adiponectin isoforms in elderly patients with or without coronary artery disease. J Am Geriatr Soc 2010;58:702-6.
- Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol 2015;136:304-11.e8.
- Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab 2014;113:155-60.
- Choi BH, Kim YH, Ahn IS, Ha JH, Byun JM, Do MS. The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling. Nutr Res Pract 2009;3:84-8.
- Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int 2014;2014:658913.
- Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006;49:394-7.
- Dwajani S, Kumar VS, Keerthi D. Atorvastatin and simvastatin as analgesic agents in experimental models. J Basic Clin Pharm 2012;3:332-5.
- Schoormans D, Mager YL, Oort FJ, Sprangers MA, Mulder BJ. New York Heart Association class assessment by cardiologists and outpatients with congenital cardiac disease: A head-to-head comparison of three patient-based versions. Cardiol Young 2012;22:26-33.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
- Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF, et al. Potential long-term effects of previous schistosome infection may reduce the atherogenic index of plasma in Chinese men. Int J Parasitol 2015;45:289-94.
- Nunes SO, Piccoli de Melo LG, Pizzo de Castro MR, Barbosa DS, Vargas HO, Berk M, et al. Atherogenic index of plasma and atherogenic coefficient are increased in major depression and bipolar disorder, especially when comorbid with tobacco use disorder. J Affect Disord 2015;172:55-62.
- Allou N, Bronchard R, Guglielminotti J, Dilly MP, Provenchere S, Lucet JC, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med 2014;42:1150-6.
- Hyun S, Li X, Vermillion B, Newton C, Fall M, Kaewprag P, et al. Body mass index and pressure ulcers: Improved predictability of pressure ulcers in intensive care patients. Am J Crit Care 2014;23:494-500.
- Joshi D, Missiuna C, Hanna S, Hay J, Faught BE, Cairney J. Relationship between BMI, waist circumference, physical activity and probable developmental coordination disorder over time. Hum Mov Sci 2015;40:237-47.
- Meller MM, Courville AB, Sumner AE. Persistently high hip circumference after bariatric surgery is a major hurdle to successful hip replacement. Case Rep Med 2014;2014:786474.
- Brydon L. Adiposity, leptin and stress reactivity in humans. Biol Psychol 2011;86:114-20.
- Maciejczyk M, Wiecek M, Szymura J, Szygula Z, Wiecha S, Cempla J. The influence of increased body fat or lean body mass on aerobic performance. PLoS One 2014;9:e95797.
- Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The heritage family study. Int J Obes Relat Metab Disord 2002;26:789-96.
- Hong KW, Chung M, Cho SB. Meta-analysis of genome-wide association study of homeostasis model assessment ß cell function and insulin resistance in an East Asian population and the European results. Mol Genet Genomics 2014;289:1247-55.
- Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatr Res 2007;61:345-9.
- Kishida K, Funahashi T, Shimomura I. Molecular mechanisms of diabetes and atherosclerosis: Role of adiponectin. Endocr Metab Immune Disord Drug Targets 2012;12:118-31.
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730-7.
- Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, et al. Adiponectin and coronary heart disease: A prospective study and meta-analysis. Circulation 2006;114:623-9.
- Phillips SA, Kung JT. Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol 2010;10:676-83.
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005;11:1096-103.
- Fu F, Zhao K, Li J, Xu J, Zhang Y, Liu C, et al. Direct evidence that myocardial insulin resistance following myocardial ischemia contributes to post-ischemic heart failure. Sci Rep 2015;5:17927.
- Ajith TA, Riji T, Anu V. In vitro anti-oxidant and DNA protective effects of the novel 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor rosuvastatin. Clin Exp Pharmacol Physiol 2008;35:625-9.
- Thongtang N, Ai M, Otokozawa S, Himbergen TV, Asztalos BF, Nakajima K, et al. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation. Am J Cardiol 2011;107:387-92.
- Nagay Hernández S, Flores Molina JJ, Ilarraza Lomelí H, Martínez Sánchez C, del Valle Mondragón L, Tenorio López FA, et al. Influence of rosuvastatin in endothelial function and oxidative stress, in patients with acute coronary syndrome. Arch Cardiol Mex 2008;78:379-83.
- Kurogi K, Sugiyama S, Sakamoto K, Tayama S, Nakamura S, Biwa T, et al. Comparison of pitavastatin with atorvastatin in increasing HDL-cholesterol and adiponectin in patients with dyslipidemia and coronary artery disease: The COMPACT-CAD study. J Cardiol 2013;62:87-94.
- Rafraf M, Mohammadi E, Asghari-Jafarabadi M, Farzadi L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr 2012;31:361-8.
- Yamamoto T, Kajikawa Y, Otani S, Yamada Y, Takemoto S, Hirota M, et al. Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: The possible involvement of adiponectin. Acta Med Okayama 2014;68:349-61.
- Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, et al. Adiponectin translation is increased by the PPAR-gamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab 2009;296:E480-9.
- Lorente-Cebrián S, Pérez-Matute P, Martínez JA, Marti A, Moreno-Aliaga MJ. Effects of eicosapentaenoic acid (EPA) on adiponectin gene expression and secretion in primary cultured rat adipocytes. J Physiol Biochem 2006;62:61-9.
- Dokholyan RS, Albert CM, Appel LJ, Cook NR, Whelton P, Hennekens CH. A trial of omega-3 fatty acids for prevention of hypertension. Am J Cardiol 2004;93:1041-3.
- Makariou SE, Liberopoulos EN, Agouridis AP, Challa A, Elisaf M. Effect of rosuvastatin monotherapy and in combination with fenofibrate or omega-3 fatty acids on serum Vitamin D levels. J Cardiovasc Pharmacol Ther 2012;17:382-6.
- Mindrescu C, Gupta RP, Hermance EV, DeVoe MC, Soma VR, Coppola JT, et al. Omega-3 fatty acids plus rosuvastatin improves endothelial function in South Asians with dyslipidemia. Vasc Health Risk Manag 2008;4:1439-47.
- Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 2010;33:1629-34.
- Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CD, Snijder MB, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab 2008;93:1489-96.
- Monroe AK, Gudzune KA, Sharma R, Chelladurai Y, Ranasinghe PD, Ansari MT, et al. Combination Therapy Versus Intensification of Statin Monotherapy: An Update. Report No. 14-EHC013-EF. AHRQ Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- Li X, Gao X, Zhang B, Zhao NQ. The relationship between adiponectin and coronary heart disease metabolic risk factors in non-diabetic male patients. Zhonghua Yi Xue Za Zhi 2007;87:1971-4.
- Wennberg AM, Gustafson D, Hagen CE, Roberts RO, Knopman D, Jack C, et al. Serum Adiponectin Levels, Neuroimaging, and Cognition in the Mayo Clinic Study of Aging. J Alzheimers Dis 2016;4;53:573-81.