Clinical Implications of Molecular PEGylation on Therapeutic Proteins
- *Corresponding Author:
- Dr. Dhanaraju Dasaratha Magharla
Department of Pharmaceutics, GIET School of Pharmacy, NH-16 Chaitanya Knowledge City, Rajahmundry, Andhra Pradesh, India.
E-mail: mddhanaraju@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Molecular PEGylation has redefined the clinical importance of many biomolecules by improving their pharmacodynamic and pharmacokinetic properties. From its inception, PEGylation has grown substantively into a well-established technology to facilitate the clinical translation of macromolecules by overcoming their limitations. PEGylation renders a number of benefits to therapeutic proteins, such as, increase in hydrodynamic size, extension of circulation half-life, prevention of proteolytic degradation and reduction of immunogenicity and antigenicity. The successful entrance of the PEGylated protein pharmaceuticals to the market, can be ascribed to the unique properties of poly (ethylene glycol) (PEG) conjugated to these proteins. This article aims to review the precise role of PEG in improving the therapeutic efficacy of PEG-protein conjugates approved by regulatory bodies. The data presented herein were extracted from articles published in peer reviewed journals, official websites of manufacturers and safety labeling and drug approval summary of the FDA Centre for Drug evaluation and Research (CDER).
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Keywords
PEGylation, proteins, poly(ethylene glycol), circulation half-life, immunogenicity
Introduction
The advent of molecule-altering technologies and improved synthetic methods has led to the finding of newer proteins and peptides that resemble human proteins and peptides.[1] Although, capable of producing potential therapeutic benefits, protein molecules have serious biopharmaceutical concerns such as, poor shelf- life, rapid degradation in the physiological environment, poor solubility, immunogenicity and antigenicity.[2] These concerns can be overcome by utilizing the beneficial properties of polyethylene glycols and PEGylation. ‘PEGylation’ is the process of chemical attachment of PEG to bioactive proteins and peptides, to modify their pharmacokinetic and pharmacodynamic properties. Here, we present a brief summary of molecular PEGylation and PEGylated proteins currently approved for the clinical management of various conditions including oncology, infectious diseases and metabolic disorders.
Background
The origin of PEGnology dates back to the 1960’s, when Davis and his colleagues were looking for a solution to overcome the immunological reactions produced injection of foreign proteins into humans. Eventually, they found that coupling monomethoxy PEGs to therapeutic proteins yielded PEG-proteins which possessed low immunogenicity and longer circulating half-lives in comparison to their unpegylated equals.[3] Then onwards PEGylation has grown profoundly into a well-accepted and well-established technology to produce biomolecules with improved stability and potency.[4]
Molecular PEGylation
The attachment of PEG to drug molecule to alter its bio-distribution, pharmacokinetics and toxicity is termed as molecular PEGylation. [5] Molecular PEGylation is principally used to configure therapeutic proteins and enzymes, although seldom used on small drug molecules. [6] PEGylating a protein or a peptide by chemical linkage is generally intended at improving its water solubility, bioavailability, decreasing elimination rate, formation of stable linkage and augmenting the therapeutic activity.[7] The most important application of PEG conjugation is increasing the circulating half-life of proteins and peptides. Research has shown that biological half-life and bioavailability of interferon-α-2a,[8] tumor necrosis factor,[9] brain derived neurotropic factor,[10] growth hormone-releasing factor,[11] asparaginase,[12] lactoferrin,[13] interleukin-2,[14] and streptokinase[15] are improved significantly after PEGylation than the native proteins. The anticancer activity of interferon-α-2a and tumor necrosis factor increased profoundly after conjugation with PEG. Though extensive research have hovered towards the advancement in PEGylated proteins, only a few products has entered the market owing to expensive costs involved in the clinical development and regulatory approval process. [16] Of the marketed nanomedicines which got approval from the regulatory bodies, nearly 40% are based on protein-polymer conjugates and liposomal formulations.[17] The list of PEGylated proteins which are approved for clinical applications is presented in Table 1.
EMA: European Medicines Agency; FDA: The Food and Drug Administration (USFDA)
Table 1: List of FDA approved PEG-Protein conjugate.
PEGylation-chemistry and disposition
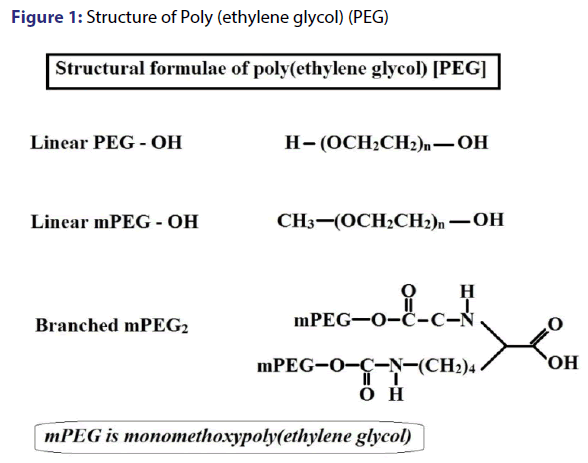
Polyethylene glycol is considered as an ideal polymer for conjugation with proteins owing to its desirable properties such as, non-toxicity, non-immunogenicity, non-antigenicity, amphiphilic nature, FDA approval and low accumulation in the reticulo-endothelial system (RES) organs.[18] PEG is an inert polymer comprising of repetitive units of ethylene oxide, either as linear or branched chains as shown in figure 1. PEGs are commercially available in different molecular weights with functional groups present at one or more termini to facilitate conjugation. PEGs with free hydroxyl groups at both ends (-OH) or PEGs with methoxylated groups (-OCH3) at one or two ends are frequently used in conjugation, of which the latter has important application in PEGylation. Reaction conditions and PEGylation chemistry are immensely responsible for the functional properties of the conjugated proteins.[19] The PEG chemistry for amine conjugation include PEG tresylate, PEG succinimidyl carbonate, PEG succinimidyl succinate, PEG dichlorotriazine, PEG trichlorophenyl carbonate, PEG p-nitrophenyl carbonate, PEG benzotriazole carbonate and PEG carbonylimidazole. PEGylation of cystein involves the utilization of thiol reactive derivatives such as PEG iodoacetamide, PEG-maleimide, PEG orthopyridyl disulfide. PEG hydrazides are much useful in conjugating oxidized carbohydrates or N-terminal serine or threonine.[1]
A PEG derivative with suitable functional chemistry is essential to couple a peptide or protein molecule and to prolong its circulatory time. This is achieved possibly by two effects; increased protection from proteolytic degradation and decreased rate of clearance of kidney. The proteolytic degradation is prevented by the stearic hindrance created by the hydrated PEG chains, which reduce the interactive collisions between reactive domains of proteins and enzymes. The decreased rate of clearance occurs in response to the dramatic increase in the hydrodynamic volume following conjugation with PEG, which in turn reduces the glomerular filtration.[20]
The following section reviews in brief, the effect of PEGylation on the clinical aspects of therapeutic PEG-protein conjugates approved for use in patients.
Adagen
Adagen is Pegademase bovine (PEG-ADA), approved by FDA in 1990, as a placement therapy for Severe Combined Immunodeficiency Disease (SCID), a genetically inherited, fatal disorder, caused by deficiency of Adenosine deaminase (ADA).[21] Decrease in the levels of ADA results in intracellular and extracellular accumulation of toxic substances such as, adenosine (Ado) and 2’-deoxyadenosine (d-Ado) and have its intense manifestation on the immune system. The successful management of SCID involves hematopoietic stem cell transplant, gene therapy and enzyme replacement therapy by red cell transfusions. Enzyme replacement therapy using PEG-ADA is not a curative therapy, but considered as an important treatment option to promote the well-being of the patient. ADA is a cytosolic enzyme, which catalyzes the conversion of Ado and d-Ado to inosine and 2-deoxyinosine, respectively in the cell location. PEG-ADA does not reach the cellular location owing to the PEG component. It can produce the same effect on the nucleosides by diffusing through the cell. The conjugation of monomethoxy polyethylene glycol (PEG) to ADA through a lysine bridge adds immense therapeutic benefits to ADA. The circulation half-life of ADA was increased from few minutes to 24 h by resisting the neutralization by circulating antibodies. PEG also protects the enzyme from proteolytic attack and renal clearance.[22]
Oncaspar
Oncaspar, the PEG conjugated version of E-coli derived asparaginase is approved as first line treatment for childhood acute lymphoblastic leukemia (ALL), in the US. Asparaginase hydrolyses plasma asparagines into ammonia and aspartic acid. Since, ALL cells lack asparagine synthetase, they depend on plasma asparagine for intracellular synthesis of asparagines. L-asparaginase prevents the protein synthesis of lymphoblasts by depleting the plasma asparagines and consequently cause the cell death.[23] The downside in the treatment with native L-asparaginase are the adverse effect on normal protein synthesis and recurrent hypersensitivity reactions in patients. PEG-asparaginase is well tolerated in patients and the advent of severe hypersensitivity reactions is uncommon.[24] The PEG component decreases the immunogenic episodes by reducing the development of anti-asparaginase antibodies.[25] PEGylation of native L-asparaginase increase its circulation half-life from 5.5 h to 1.2 days.[26]
Pegvisomant
Pegvisomant - a genetically engineered antagonist of growth hormone (GH) approved for the treatment and management of acromegaly is another protein in which the role of PEG is well exhibited. Acromegaly is a rare hormonal disorder characterized by the excess production of growth hormone (GH) by pituitary adenoma, which leads to increased secretion of insulin like growth factor I (IGF-I). The treatment for acromegaly is reliant on removal of adenoma or regulation of GH/IGF-I levels. Pegvisomant inhibits the metabolic effects of the hypersected GH and normalizes the levels of IGF-I. Pegvisomant acquires its antagonistic effects due to one of the nine amino acids (G120K), while the rest is responsible for the binding to growth hormone receptor (GHR).[27] PEGylation of the GH analogue increase its half-life to 100 h and binding affinity to GH.[28] PEGylated GH analogue can prolong the efficacy up to 12 weeks due to the sustained suppression of IGF-I.[29]
Pegasys and Peg-intron
PEGylated Interferon α-2a (Pegasys) and α-2b (Peg-intron) were approved by FDA in 2002 and 2001, respectively, as the first line treatment for Chronic Hepatitis C infection. The infection of Hepatitis C virus (HCV) was dealt previously with Interferon-alpha (INF-α) monotherapy or with a more effective combination therapy using INF-α and broad spectrum antiviral agent, ribavirin. INF-α inhibits virus protein translation and destabilizes viral RNA by inducing interferonstimulated genes (ISG) through INF receptor- JAK/STAT mediated pathway.[30] The shortcoming of INF-α treatment in its unmodified form is its short half-life, frequent administration and fluctuating plasma levels. The plasma half-life of INF-α2a is 4-6 h which is reduced to undetectable levels within one day following subcutaneous injection, persuading the administration of three doses per week. The fluctuating levels of INF α-2a in plasma during this treatment regimen pave way for entry and replication of virus. PEGylation of INF-α2a using a branched 40 kDa PEG prolonged the half-life from 3.8 h to 65 h and delayed the clearance by 100 fold. Phase III clinical trials demonstrated that the effectiveness of PEG-INF-α2a, given subcutaneously, once a week is superior to INF-α2a, given subcutaneously 3 times per week, which could be ascribed to the increased circulation half-life and reduced existence of virus particles.[31] Similarly, PEGylation of INF- α2b increases the half-life of native protein from 7 h to 48 h and the recommended dose of administration of Peg-intron is 1 mg/kg per week for 52 weeks, due to its prolonged effect.[32]
Pegaptanib
Pegaptanib sodium is an RNA aptamer used as an anti-vascular endothelial growth factor (anti-VEGF) directed therapeutic agent for the treatment of neovascular age related macular degeneration (AMD). The degeneration of macula following metabolic stresses in AMD results in loss of central vision. Pegaptanib was approved by the US FDA in 2004 and is the first aptamer used for clinical application. Aptamers are oligonucleotides that bind biological proteins with high affinity and specificity. Aptamers are preferred over the naturally occurring DNA or RNA molecules as they interact with molecular targets using superior binding abilities and adaptive recognition capabilities even at picomolar or nanomolar concentrations.[33] VEGF is the principal regulator of vascular permeability and pathological angiogenesis and hence selected as the target for the development of pegatanib in ocular neovascular diseases.[34] Pegaptanib sodium (1 mg/3 mg) is administered by intravitreous route, every six weeks for 48 weeks. The traditional topical delivery of pegaptanib may not produce the clinical benefits in posterior chamber diseases like AMD, as they increase the therapeutic concentrations in the anterior chamber of the eye but not the posterior chamber. The intravitreal injection facilitates the drug to interact with VEGF, responsible for the choroidal neovascularization in the posterior chamber. Conjugation of PEG (40 kDa) to the aptamer reduces its binding affinity to VEGF by four folds, but prolongs the residence time and maximizes the efficacy by slowing down the diffusion out of the vitreous humor.[29] PEGylation minimizes the systemic effects and the occurrence of adverse events is transient to moderate and is mostly related to the injection procedure rather than the drug itself.
Certolizumab Pegol
Certolizumab Pegol is PEGylated anti-Tumor necrosis factor-alpha (TNF-α), approved for the treatment of Crohn’s disease (CD) and rheumatoid arthritis (RA). It is an antigen binding fragment (Fab’) of a monoclonal antibody that accede to conjugation with PEG which in turn enhances the pharmacokinetic properties and reduced immunological reactions.[35] TNF-α is a cytokine involved in the pathogenesis of autoimmune diseases including CD and RA. Therefore, decreasing the levels of TNF-α could improve the conditions of the above said immunological diseases.[36] Certolizumab pegol is given alone or in combination with methotrexate in RA and as a monotherapy for CD. It neutralizes both soluble and transmembrane TNFα and produce a longer and effectual effect on the symptoms of these diseases over its TNF-α monoclonal antibody counterpart (natalizumab, adalimumab and infliximab). The better clinical outcomes of Certolizumab pegol can be due to the lack of Fc region which reduces the Fc mediated response viz., antibody dependent, cell-mediated and complementmediated cytotoxicity.[37,38] Also, the conjugation of a 40 kDa PEG molecule increases the stability of Certolizumab, which facilitate the administration of the drug by subcutaneous route instead of intravenous route. PEGylation alters the pharmacokinetics of anti- TNF-α by improving its distribution and penetration into inflamed tissues preventing the TNF-α induced inflammation.[39]
Pegfilgrastim
Cytotoxic chemotherapy induced neutropenia is a serious condition in which the host hematopoietic system is suppressed, compromising the immune mechanism of the body. Thus, neutropenia is associated with the episodes of life-threatening infections, intolerance to chemotherapy dose and impediment in the expected clinical outcomes. Neutrophils are key components of innate immunity and are responsible for the protection against infections. Chemotherapy suppresses the production of neutrophils; as a result, patients with neutropenia show reduced signs and symptoms with fever being the only sign of infections.[40] Pegfilgrastim (Neulasta) was approved in 2002, for the treatment of febrile neutropenia.. Pegfilgrastim is formed by conjugating a 20 kDa monomethoxy polyethylene glycol aldehyde to Granulocyte-Colony Stimulating Factor G-CSF.[41] PEGylation increases the half-life to 42 h against 3.6 h of non-PEGylated G-CSF, which reduce the frequency of drug administration. Pegfilgrastim offers lower elimination rate and less incidence of febrile neutropenia compared to filgrastim.[42]
Pegloticase
Pegloticase (Krystexxa) is PEGylated mammalian urate oxidase approved in 2010 for the treatment of chronic gout in adult patients, refractory to conventional therapy. Pegloticase catalyze the oxidation of uric acid to allantoin-an inert, water soluble metabolite and thereby reduce serum uric acid levels. The inflammation and pain caused due to uricase crystal formation in plasma can be prevented by Pegloticase. It is more effective in treating gout over other available treatments.[43] The PEGylation using 20 kDa to uricase tend to reduce immunogenicity reactions and increases its half-life from eight hours to twelve days.[29]
Methoxy polyethylene glycol-epoetin beta
Anemia associated with chronic kidney disease (CKD) is a major cause of morbidity which, if untreated, can lead to more devastating conditions including deterioration of cardiac function. Frequent red cell transfusions was the only treatment available to combat anemia in CKD and hemodialysis patients, before the introduction of erythropoietin stimulating agents (ESA).[44] First generation ESAs (Human recombinant erythropoietins-alpha and beta) and second generation ESAs (darbepoetin Alfa and continuous erythropoietin receptor agonist (CERA)) have greatly improved the quality of life of CKD patients over two decades.[45] Methoxy polyethylene glycolepoetin beta (Mircera) is approved for the treatment of patients with anemia associated with CKD in 2007. It is synthesized by the addition of activated monomethoxy PEG butanoic acid to lysine 46 and 52 on erythropoietin.[46] The molecular weight of Mircera is 60 kDa, of which the polyethylene glycol moiety accounts for 30 kDa. Increase in the glycosylation by the conjugation of PEG offered longer half-life (130 h) and improved biological activity compared to first generation erythropoietins.[47] Mircera requires once monthly administration and produces negligible adverse reactions, thereby, considerably improves patient compliance than any other ESAs.[21]
Peginesatide
Peginesatide (Omontys) is an erythropoietin stimulating agent (ESA) developed by Affymax and Takeda. This functional analog of erythropoietin was approved by The US FDA in 2012, for anemia treatment associated with CKD in adult patients on dialysis. Peginesatide is a synthetic peptide consisting of 21 amino acids bonded together by a linker derived from β-alanine and iminodiacetic acid. This dimeric peptide molecule is attached to a single lysine-branched bis- (methoxypolyethylene glycol) (MW=40 kDa) to improve its biological half-life and reduce immunogenic reactions. Peginesatide binds to erythropoietin receptor and facilitates erythroblast proliferation resulting in increased reticulocyte and RBC count. The outcomes of Phase III clinical trials showed that Peginesatide is not inferior to standard ESA agents in improving hemoglobin levels within the target range. However, PEARL (Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis) study demonstrated that the safety endpoint of cardiovascular events (unstable angina, and arrhythmia) and death was inferior for peginesatide than darbepoetin. The rate of renal failure was twice as high in patients receiving peginesatide. Though there is no clear justification for these adverse reactions encountered by patients, peginesatide was voluntarily recalled from the market by its manufacturer in 2013.[43]
Molecular PEGylation of small drug molecule
Research has shown that biologically active drugs, ibuprofen, aspirin, benzoylacrylic acids, and ursodeoxycholic acid, on conjugation with PEG esters exhibited better therapeutic benefits over unmodified molecules. However, the PEG-low molecular weight drug molecule conjugates are yet to be approved for clinical use. Of those that entered clinical trials, the four important products which are at various phases of clinical trials are PEG-Camptothecin (Prothecan), PEG-Naloxol (NKTR-118), PEG-Irinotecan (NKTR-102), PEG-SN38- a metabolite of irinotecan (EZN-2208). The conjugation of the PEG moiety with small organic drugs is much simpler as compared to macromolecular PEGylation. As these molecules possess less functional groups and free from conformational limitations, purification and characterization of PEGylated-drug conjugates is done at ease.[2,4]
Conclusion
PEGylation is a versatile technology capable of altering the properties of both bioactive molecules and drug loaded particulate carrier systems. In the former case, molecular PEGylation could impart distinguished pharmacokinetic properties and benefits to proteins and small drug molecules over their biosimilars. In the latter situation, PEGylation renders steal thing abilities to drug carriers, such as liposomes, polymeric nanoparticles, metal nanoparticles, lipid emulsions, etc. The PEG molecules adhered or grafted on the surface of these drug carriers camouflages them from interactions with plasma proteins and subsequent clearance by the mononuclear phagocytic system (MPS). Therefore, PEGylation is an essential technique to modify the physicochemical properties of the parent drug either free form or encapsulated form to improve its immunogenicity, cellular uptake, spatial placement and biological activity. The scope of PEGylation in the near future is still intensifying as it is the most reliable tool available to help the innovative medicinal products meet the clinical and regulatory standards.
References
- Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. AdvDrugDeliv Rev 2002;54:459-76.
- Kang JS, Deluca PP, Lee KC. Emerging PEGylated drugs. Expert OpinEmerg Drugs 2009;14:363-80.
- Davis FF. The origin of pegnology. Adv Drug Deliv Rev 2002;54:457-8.
- Li W, Zhan P, De Clercq E. Current drug research on PEGylation with small molecular agents. Prog Polym Sci 2013;38:421-44.
- Duncan R, Kopecek J. Soluble synthetic polymers as potential drug carriers. AdvPolym Sci 1984;57:53-101.
- Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of PEGylation. Drug Deliv 2006;13:399-409.
- Harris JM, Martin NE, Modi M. PEGylation: a novel process for modifying pharmacokinetics. ClinPharmacokinet 2001;40:539-51.
- Chatelut E, Rostaing L, Grégoire N. A pharmacokinetic model for alpha interferon administered subcutaneously. Br J ClinPharmacol 1999;47:365-71.
- Tsutsumi Y, Kihira T, Tsunoda S. Molecular design of hybrid tumor necrosis factor alpha with polyethylene glycol increases its anti-tumour potency. Br J Cancer 1995;71:963-8.
- Pardridge WM, Wu D, Sakane T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotropic factor following intravenous administration. Pharm Res1998;15:576-82.
- Campbell RM, Heimer EP, Ahmad M. Pegylated peptides. V. Carboxy-terminal PEGylated analogs of growth hormone-releasing factor (GRF) display enhanced duration of biological activity in vivo.J Pept Res 1997;49:527-37.
- Holle LM. Pegaspargase: an alternative? Ann Pharmacother 1997;31:616-24.
- Beauchamp CO, Gonias SL, Menapace DP. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and alpha 2-macroglobulin. Anal Biochem 1983;131:25-33.
- Knauf MJ, Bell DP, Hirtzer P. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J BiolChem 1988;263:15064-70.
- Rajagopalan S, Gonias SL, Pizzo SV. A nonantigenic covalent streptokinase-polyethylene glycol complex with plasminogen activator function.J Clin Invest 1985;75:413-9.
- Sainz V, Conniot J, Matos AI. Regulatoryaspects on nanomedicines.BiochemBiophys Res Commun 2015;468:504-10.
- Gaspar R, Florindo H, Silva L. Regulatory aspects of oncologicals: nanosystems main challenges. In: Alonso MJ, Garcia-Fuentes M (eds).Nano-oncologicals. Springer International Publishing 2014;pp:425-452.
- Brocchini S, Godwin A, Balan S. Disulfide bridge based PEGylation of proteins. Adv Drug Deliv Rev 2008;60:3-12.
- Kinstler OB, Brems DN, Lauren SL. Characterization and stability of N-terminally PEGylated rhG-CSF. Pharm Res 1996;13:996-1002.
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003;2:214-21.
- Banerjee SS, Aher N, Patil R. Poly(ethylene glycol)-Prodrug Conjugates: Concept, Design, and Applications. J Drug Deliv 2012; ID 103973. Available from: https://www.hindawi.com/journals/jdd/2012/103973/
- Booth C, Gaspar HB. Pegademase bovine (PEG-ADA) for the treatment of infants and children with severe combined immunodeficiency (SCID).Biologics 2009;3:349-58.
- Graham ML. Pegaspargase: Areview of clinical studies. Adv Drug Deliv Rev 2003;55:1293-02.
- Douer D, Yampolsky H, Cohen LJ. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adultsaged55years or younger with newly diagnosed acute lymphoblastic leukemia. Blood 2007;109:2744-50.
- Mondelaers V, Bauters T, de Moerloose B. PEG-asparaginase in the treatment of childhood acute lymphoblastic leukaemia. Belg J Hematol 2013;4:138-43.
- Fu CH, Sakamoto KM. PEG-asparaginase. Expert OpinPharmacother 2007;8:1977-84.
- Bernabeu I, Rodriguez-Gomez IA, Ramos-Levi AM. Profile of pegvisomant in the management of acromegaly: an evidence based review of its place in therapy. Res Rep EndocrDisord 2015;5:47-58.
- Kopchick JJ, Parkinson C, Stevens EC. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 2002;23:623-46.
- Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci 2008;97:4167-83.
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C.Nature 2005;436:967-72.
- Zeuzem S, Feinman SV, Rasenack J. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;343:1666-72.
- Bukowski RM, Tendler C, Cutler D. Treating cancer with PEG Intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer 2002;95:389-96.
- Hermann T, Patel DJ. Adaptive recognition by nucleic acidaptamers. Science 2000;287:820-5.
- Ng EW, Shima DT, Calias P.Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006;5:123-32.
- Baka S, Clamp AR, Jayson GC.A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert OpinTher Targets 2006;10:867-76.
- Tracey D, Klareskog L, Sasso EH. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. PharmacolTher2008;117:244-79.
- Nesbitt A, Fossati G, Bergin M. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumour necrosisfactoraagents.InflammBowelDis 2007;13:1323-32.
- Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. TherapAdvGastroenterol 2011;4:375-89.
- Eddleston A, Marenzana M, Marshall D. Comparison of the distribution of an IGG and a pegylated Fab? form of an anti-TNF a antibody in the inflamed gut of colitic mice. Gut 2009;15:A305.
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004;100:228-37.
- Green MD, Koelbl H, Baselga J. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003;14:29-35.
- Molineux G. Pegfilgrastim: Using pegylation technology to improve neutropenia support in cancer patients. Anticancer Drugs 2003;14:259-64.
- Hershfield MS, Sundy JS, Ganson NJ. Development of PEGylated mammalian urate oxidase as a therapy for patients with refractory gout. In: Veronese FM (ed). PEGylated Protein Drugs: Basic Science and Clinical Applications. Switzerland: Birkhauser; 2009;217-27.
- Kaushik T, Yaqoob MM. Lessons learned from peginesatide in the treatment of anemia associated with chronic kidney disease in patients on dialysis. Biologics 2013;7:243-6.
- Kiss Z, Elliott S, Jedynasty K. Discovery and basic pharmacology of erythropoiesis-stimulating agents (ESAs), including the hyperglycosylated ESA, darbepoetin alfa: an update of the rationale and clinical impact. Eur J ClinPharmacol 2010;66:331-40.
- Macdougall IC, Eckardt KU. Novel strategies for stimulating erythropoiesis and potential new treatments for anemia. Lancet 2006;368:947-53.
- Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. CurrHematol Rep 2005;4:436-40.