Anti-Inflammatory and Anti-Nociceptive Activities of Stem-Bark Extracts and Fractions of Carpolobia Lutea (Polygalaceae)
- *Corresponding Author:
- Dr. Lucky Legbosi Nwidu
Harruk Road, Rumuigbo Town, Port Harcourt, Rivers State, Nigeria.
E-mail: menelucky@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: In Niger Delta, ethnomedicine hydroalcoholic extract of Carpolobia lutea (CL) (Polygalaceae) is used to relieve inflammatory pains. Objectives: The purpose of this study is to evaluate the antiâÂÂinflammatory and antinociceptive effects of ethanolic stem extract (ESE) and to fractionate the ESE for the elucidation of bioactive molecules. Materials and Methods: The antinociceptive effects for ESE were tested against two noxious stimuli; chemical (acetic acidâÂÂinduced writhing and formalinâÂÂinduced pain) and thermal (hot plate) stimuli. The effects of paracetamol (130 mg/kg), indomethacin (10 mg/kg), and morphine (5 mg/kg) pretreatment were investigated. To isolate the bioactive compounds with antiâÂÂinflammatory effect, two doses (86.6 and 173.2 mg/kg) of four fractions (methanol fraction MTF, ethyl acetate fraction EAF, chloroform fraction CHF, and n-hexane fraction n-HF) obtained from fractionating ESE were utilized. Carrageenan, egg albumin, and capsaicinâÂÂinduced edema of the hind paw of the rats were the models adopted. Paw volume was measured by a digital vernier caliper from 0 to 6 h after injection. This was compared to standard drugs. The results were subjected to statistical analysis. Results: The ESE decreased significantly (P < 0.001) the writhing of acetic acidâÂÂinduced abdominal contractions and licking of formalinâÂÂinduced pains but does not have any effects on the hot plate test. Of the four fractions obtained, the EAFs demonstrated a significant (P < 0.001) inflammatory inhibition of 98.97% and 41.91% at 86.6 and 173.2 mg/kg, respectively, compared to 65.75% inhibition demonstrated by the reference drug, acetylsalicylic acid (100 mg/kg) on the carrageenan model while 36.36% and 29.87% inhibition of inflammation at 86.6 and 173.2 mg/kg, respectively, on the egg albumin models; there was no significant effect on the capsaicin model. Conclusion: The isolation of quercetin and kaemferol from CL gave credence to its antiâÂÂinflammatory and antinociceptive effects.
Keywords
Anti-inflammatory, antinociceptive, Carpolobia lutea, kaemferol, quercetin, stem fractions
Introduction
Most diseases are accompanied by inflammation. Chronic inflammation of the brain underlies many neurodegenerative diseases, including Alzheimer’s disease, [1] prion diseases, [2] Parkinson’s disease, [3] multiple sclerosis (MS), [4] and stroke. [5] Similarly, in the gastrointestinal tract, inflammatory diseases often manifest as colic pain, cramps, and stomach upsets of varying degree. [6] Acute or chronic abdominal inflammatory pains may occur due to invasion of the gastrointestinal tract by Escherichia coli or other opportunistic infections mediated through plasmid-encoded invasion factors. These organisms are known to release enterotoxins which induce inflammation in the form of abdominal tissue damage and watery diarrheal. [6]
Besides many chronic diseases, ulcerative colitis or inflammatory bowel diseases, rheumatoid arthritis, and psoriasis exhibit inflammatory and/or immunological conditions. They are treated symptomatically due to lack of drugs for effective cure. Catalogs of drugs with diverse therapeutic effects are marketed for the treatment of inflammatory diseases such as corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and the recently marketed selective cyclooxygenase-2 (COX-2) inhibitors. Corticosteroids are significant in the treatment of inflammatory diseases but for their severe side effects/toxicity, they are reserved for only acute conditions. NSAIDs remain the fundamental anti-inflammatory drug therapy. However, these drugs are often not sufficiently effective in cases of chronic inflammation and are prone to cause severe gastrointestinal side effects such as ulcers and gastrointestinal bleeding. Thus, remedies for a number of severe inflammatory diseases are scarce despite speedy progress in medical research over the past decades. This observation has led to extreme search for plant-based products for inflammatory conditions. Plants are utilized in traditional medicine to treat patients and this practice spanned over thousands of years. [7] This is because plant extracts possess potent biochemical molecules which are used as an important component in phytomedicine. [8,9] The pharmacological and therapeutic effects originate from armamentarium of potent bioactive agents or combinations of secondary products or biomolecules existing in many plant parts such as the leaves, the stem, root, flowers, fruits, and seeds which are putative as anti-inflammatory agents. Anthraquinone, [10] phenolic compounds, [11] flavonoids, [12-15] tannins, [16] and alkaloids [17] possess anti-inflammatory activities, hence the continuous pharmacological screening of different parts of the plant to elucidate potential molecules.
Carpolobia lutea (CL) is widely reported in South-West, South-South, and North-East Nigeria and other West African countries in the treatment of various ailments, including fever, inflammations, and stomach pains. Reports on the biological screening of the leaf extracts and fractions of CL have been reviewed [18] as gastroprotective, [19] antinociceptive, [20] antidiarrheal, [21] antimicrobial, [22] anti-inflammatory, [23] neuropharmacological, [24] and antiulcer effects; [25] isolation of cinnamoyl 1-deoxyglucosides and cinnamic acid derivatives [26] from the leaves of CL has also been reported. No pharmacological investigations on the anti-inflammatory properties of the stems and stem-bark extract exist to our knowledge in any literature to repudiate or acknowledge ethnomedicinal claim. In the present study, we scrutinize the anti-inflammatory and antinociceptive effects of ethanolic stem-bark extract (ESE) and fractions of CL using acute models of inflammation in rats.
Materials and Methods
Drugs and chemicals
Acetylsalicylic acid 300 mg (Disprin®, Reckitt Benckiser Healthcare, UK), Carrageenan (Sigma-Aldrich, Bombay, India), ethanol (99.8%), tween 80 (10%), and chloroform (Sigma-Aldrich, India) were used in this study.
The ESE (500 mg) was weighed with an analytical balance and was put into a sterile container, and 5 ml of distilled water was added in aliquot and the container was corked and shaken to dissolve the extract. This procedure was repeated until the extract completely dissolved resulting in a stock solution of 100 mg/ml (0.1 g/ml) which was used for the experiment.
Plant material and extract
The stems and stem barks were collected from Itak Ikpa village in Ibibo Local Government Area of Akwa Ibom State by an Herbalist named Mr. Okon Etefia attached to Pharmacognosy Department in the University of Uyo. The plant was identified by a Botanist named Dr. (Mrs) Margret Bassey of Botany Department in the University of Uyo. A voucher specimen (UUH 998) was deposited at the University Herbarium. The stem barks were air-dried and powdered with pestle and mortar. The pulverized stem barks were stored at room temperature until used.
The stem of CL G. Don was harvested from the wild; the sample was air-dried, and powdered thereafter extracted by immersion in ethanol 70%. The powdered stem and stem-bark (500 g) were soaked in l L of ethanol. After immersing for 72 h, it was filtered with Whatman filter paper (pore sizes - 20–25 μ). The residue obtained was air-dried for 24 h and thereafter subjected to the same procedure for three successive times. The filtrate of ethanol solvent was reduced in volume nearly to dryness in a rotatory evaporator (BUCCHI USA) at 40°C, after which the extract was dried under a flow of nitrogen until constant weight was obtained. The yield was 43.4% dried extract and 15.6% oil.
Phytochemical screening
The CL and fractions were quantitatively assayed for the presence of phytochemicals such as saponins, tannins, alkaloids, terpenoids, cardiac glycosides and anthraquinone using standard procedures.
Fractionation and isolation of compounds 1 and 2
The crude ESE of CL (60 g) in aliquot (15 g × 4) was fractionated by mixing with 60 g of silica gel and eluted with 500 ml each of n-hexane, chloroform, ethyl acetate, and methanol sequentially yielding 19.09%, 8.88%, 1.75%, and 52.58%, respectively. The four fractions were reduced in volume and dried under a flow of nitrogen and thereafter stored in an air-tight container which is kept in a refrigerator until used.
A portion of the n-butanol soluble part (1.5 g) of the ethanolic crude extract of the stem-bark of CL was packed in a column (50 cm × 3 cm) and eluted in a gradient manner with dichloromethane and methanol mixtures: 99:1, 98:2, 97:3, 96:4, 95:5, 90:10, 80:20, 70:30, 60:40, 50:50, 30:70, 10:90, and methanol (100%). Aliquots (50 ml) were collected, and the progress of separation was monitored on thin layer chromatography (TLC) using the solvent systems: ethyl acetate:dichloromethane (3:2) and ethyl acetate: methanol:water (100:16.5:13.5). Fractions eluted with 5% methanol in dichloromethane were pooled together based on their TLC profile. This fraction (0.15 g) was subjected to gel filtration over Sephadex LH-20 eluting with pure methanol to afford compound 1, a yellow solid (4 mg).
The ethyl acetate-soluble portion of the ethanol extract of the bark was subjected to gel filtration over Sephadex LH-20 eluting with pure methanol, 5 ml aliquots were collected to give 65 fractions. Compound 2 was isolated from fractions 57–60 and it was a yellowish-brown solid (3.5 mg). Both compounds were subjected to spectral analysis.
Animals
Wistar rats (150–160 g) of both sexes were obtained from the Niger Delta University Animal House. All the animals were housed in standard cages under standard laboratory condition and animals were fed with standard pellet feeds (Vita feed®, Ibadan). The experiments were carried out between June 2012 and August 2013. The experimental protocol was approved by the Faculty of Pharmacy, Niger Delta University Institutional Animal Care and Use Committee (NDUAEC) on March 22, 2014, following the guidelines of the committee for the purpose of control and supervision of experimental animals.
Toxicological assays
The LD50 of the ESE of CL was determined according to the procedure described by Lorke. [27] Albino mice (20–30 g) of either sexes were used. This method involved an initial dose-finding procedure, in which the animals were divided into eight groups of three animals per group. Doses of 10, 100, 500, 1000, 1500, 2000, 3000, and 4000 mg/kg were administered intraperitoneally per animal. The treated animals were monitored for 24 h for mortality and general toxicity behavioral characteristics. From the results, four different doses of 500, 1500, 3000, and 4000 mg/kg were chosen and administered intraperitoneally to four groups of three mice per group. The treated animals were again monitored for 24 h. The LD50 was then calculated as the square root of the multiplication of the least dose that kill all the animals and the highest dose that does not kill any animal or the geometric mean of the lowest dose causing death and the highest dose causing no death, that is, LD50= (highest dose causing no death × lowest dose causing death) 1/2.
Anti-inflammatory pharmacological assay Carrageenan test
The test of carrageenan-induced rat paw edema was done according to previously reported technique of Winter et al. [28] Rats (150–160 g) were divided into six groups, each consisting of six rats per group and were treated as follows: Group 1 received 10 ml/kg tween 80 (10%) as vehicle-treated control group. Groups 2–4 received 43.3, 86.6, and 173.2 mg/kg of crude CL ESE while Groups 5-8 each received 86.6 mg/kg of methanolic fraction (MTF), ethyl acetate fraction (EAF), chloroform fraction (CHF), and n-hexane fraction (n-HF), Groups 9–12 each received 173.2 mg/kg of MTF, EAF, CHF, and n-HF of CL, and Group 13 received acetyl salicylic acid (100 mg/kg intraperitonial [i.p.]) as positive control rats. Paw volume was measured immediately after carrageenan injection and at 1–6 h intervals after the administration of the edematogenic agent using a digital vernier caliper (Christools®, Germany). Anti-inflammatory activity was assessed on the basis of inhibition of paw edema induced by the injection of 0.1 ml 0.2% carrageenan (an edematogenic agent) into the subplantar region of the right hind paw of the rats. [28]
Egg albumin test
Acute inflammation was induced by injecting 0.1 ml of fresh egg albumin on the subplantar surface of the right hand paw of rats fasted for 24 h using the technique of Winter et al. [28] Rats (160–170 g) were divided into six groups, each consisting of six rats per group and were treated as follows: Group 1 received 10 ml/kg tween 80 (10%) as vehicle-treated control group. Groups 2–4 received 43.3, 86.6, and 173.2 mg/kg of crude CL ESE while Groups 5-8 each received 86.6 mg/kg of MTF, EAF, CHF, and n-HF, Groups 9–12 each received 173.2 mg/kg of MTF, EAF, CHF, and n-HF, and Group 13 received acetyl salicylic acid (100 mg/kg i.p.) as positive control rats. Paw volume was measured immediately after egg albumin injection and at 1–6 h intervals after the administration using digital vernier caliper (Christools®, Germany). Anti-inflammatory activity was assessed on the basis of the inhibition of paw edema induced by the injection of 0.1 ml egg albumin.
Capsaicin test
Increase in rat hind paw linear circumference induced by subplantar injection of a phlogistic agent was used as the measure of acute inflammation. [29] The phlogistic agent employed in this study was capsaicin (Basra et al., 1996). Adult Wistar rats of either sex (150–200 g) were used after 24 h fasting and deprived of water only during the experiment. Inflammation of the hind paw was induced by injecting 0.1 ml of capsaicin (5 μg/kg) dissolved in 10% tween 80 into the subplantar surface of the right hind paw. The procedure is the same as evaluated for carrageenin above. Average edema was calculated following the procedure of Okoli et al. [30] and Iwueke et al. [31] (2006).
Antinociceptive assay
Acetic acid-induced writhing test
The response to i.p. injection of 0.6% acetic acid, constriction of abdominal muscle, and stretching of limb (known as writhing syndrome) was induced following the procedure described previously. [20] Wistar strain of albino mice of either sex was randomly divided into six groups, each consisting of six mice and were treated as follows: group 1 received distilled water (10 ml/kg) as vehicle-treated group, Groups 2–4 received ESE of CL (43.3, 86.6, and 173.2 mg/kg, respectively), Group 5 received paracetamol (130 mg/kg), and 15 min later, ESE of CL (86.6 mg/kg) i.p. while Group 6 received paracetamol (130 mg/kg) only. The drugs were administered 1 h before the injection of acetic acid. After the challenge, the animals were placed in pair in observation boxes, and the number of writhings was counted cumulatively over a period of 6–30 min. Antinociceptive activity was expressed as the reduction of abdominal constrictions. The percentage analgesic activity (PAA) was calculated using the following formula:
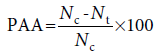
where Nc is the average number of stretches of control group and Nt is the average number of stretches of test drug group.
Formalin test
The procedure for the formalin test was essentially similar to the procedure executed previously by Nwidu et al. [20] Albino mice of either sex weighing 20–30 g were randomly divided into six groups, each consisting of six mice and were treated as follows: group 1 received distilled water (10 ml/kg) as vehicle-treated group, Groups 2–4 received ESE of CL (43.3, 86.6, 173.2 mg/kg, respectively), Group 5 received indomethacin (10 mg/kg), and 15 min later, ESE of CL (86.6 mg/kg) i.p. while Group 6 received indomethacin (10 mg/kg) only. The animals were pretreated with samples 1 h before being challenged with buffered formalin, and the responses were observed for 30 min. All mice used were injected with 2 μl of 2.5% solution of formalin (0.9 mM KCL, 2.7 mM and phosphate buffer 10 mM)subcutaneously under the surface of the right hind paw. The duration of time spent in paw licking was used as an index of nociception. The first phase (early phase) of nociceptive response normally peaked at 5 min after formalin injection and the second phase (later phase) at 20–25 min after formalin injection, representing neurogenic and inflammatory pains responses, respectively. The PAA was calculated using the following formula:
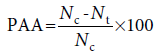
where Nc is the average number of paw licking of control group for each phase and Nt is the average number of stretches of test drug group.
Hot plate
The effect of ESE on hot plate-induced nociception in adult mice was carried out following earlier procedure executed by Nwidu et al. [20] The hot plate test was used to measure the response latencies. In these experiments, hot plate apparatus (STUART, Model-SD 500, UK) was maintained at 55°C. Albino mice of either sex weighing 20–30 g were randomly divided into six groups, each consisting of six mice and were treated as follows: group 1 received distilled water (10 ml/kg) as vehicle-treated group, Groups 2–4 received ESE of CL (43.3, 86.6, and 173.2 mg/kg, respectively), Group 5 received morphine (10 mg/kg), and 15 min later, ESE of CL (86.6 mg/kg) i.p. while Group 6 received morphine (5 mg/kg) as positive control mice. After 1 h of pretreatment with drugs or extract, the animals were placed into a beaker of 50 cm diameter on the heated surface of hot plate kept at a temperature of 65°C for a maximum time of 30 s to prevent tissue damage. Reaction time was recorded as the duration between placement and shaking or licking of fore and hind paw, or jumping which was recoded as the index of response latency. An automatic 30 s cutoff was used to prevent tissue damage. The PAA was calculated using the following formula:
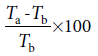
where Ta is the reaction time allowing the administration of the extract and morphine and Tb is the initial reaction time (mean reading of reaction time of control group).
Statistical analysis and data evaluation
Data obtained from this work were analyzed statistically using Student’s t-test and by multiple comparisons of mean ± standard error of the mean by one-way and two-way analysis of variance (one- or two-way) followed by a post test (Turkey–Kramer multiple comparison test). A probability level of <5% was considered statistically significant (P ≤ 0.05).
Results
Phytochemical screening
Preliminary phytochemical screening of the ESE of CL revealed the presence of alkaloids = cardiac glycosides = saponins = tannins > anthraquinone [Table 1]. Further fractionation of the crude ESE revealed that the MTF contains alkaloids = saponins > cardiac glycosides = tannins = anthraquinone; CHF shows anthraquinone > saponins = cardiac glycosides = tannins = alkaloids; n-HFs reveal cardiac glycosides = saponins > anthraquinone = tannins = alkaloids; EAF indicate saponins = anthraquinone > alkaloids = cardiac glycosides = tannin. Compound 1 and 2 were isolated from the CL and EAF respectively [Table 1].
| Phytochemicals | C. lutea stem-bark | ||||
|---|---|---|---|---|---|
| Fractions | Extract | ||||
| MTF | CHF | n-HF | EAF | ESE | |
| Alkaloids | ++ | + | + | + | ++ |
| Cardiac glycosides | + | + | ++ | + | ++ |
| Saponins | ++ | + | ++ | ++ | ++ |
| Tannins | + | + | + | + | + |
| Anthraquinone | + | ++ | + | ++ | + |
ESE: Ethanolic stem-bark extract, MTF: Methanol Fraction of stem-bark at stated doses, CHF: Chloroform fraction, n-HF: n-Hexane Fraction, EAF: Ethyl acetate Fraction (+): detected in moderate quantity, (++): Detected in abundant quantity
Table 1: Phytochemical constituents of C. lutea stem bark Extract and fractions
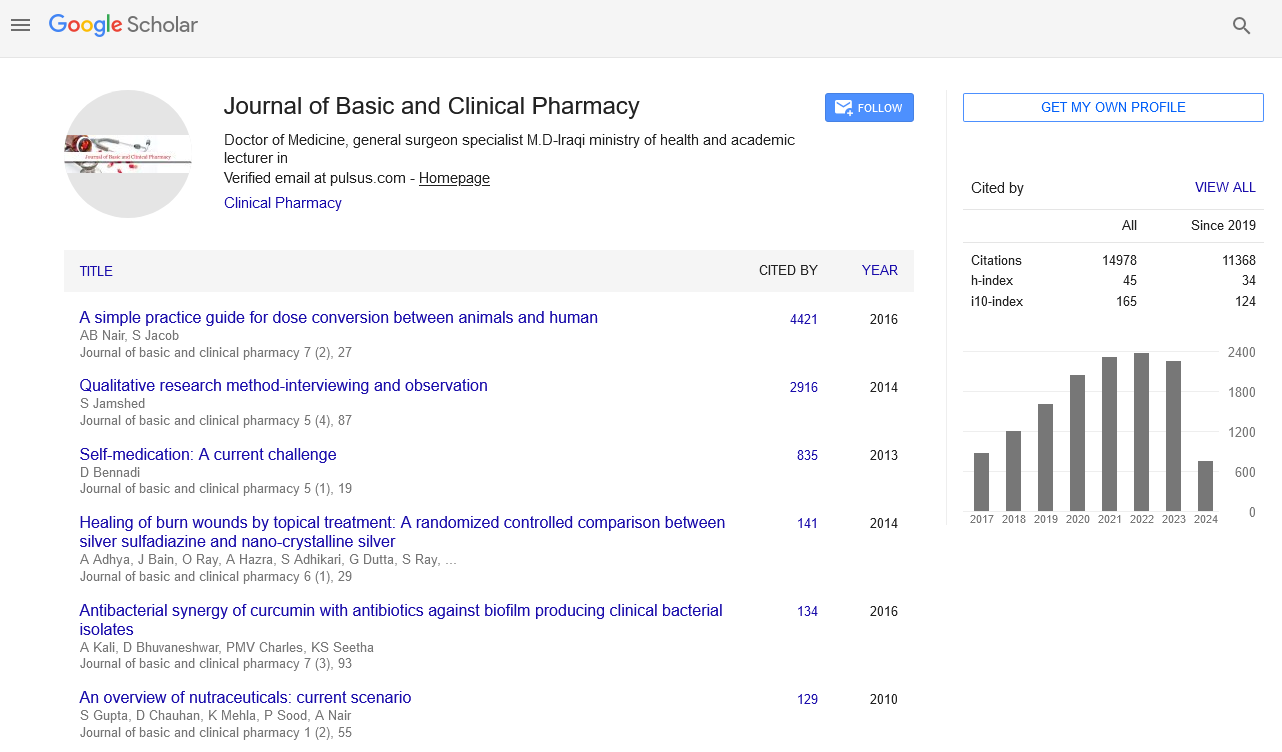
Isolation of compound 1 and 2
Compound 1 [Figure 1] is a yellow amorphous solid (4 mg). Ultraviolet (UV) (MeOH) is 256 nm and 354 nm. 1H-NMR (CD3OD) ð (ppm): 6.22 1H (d; J = 2 Hz); 6.40 1H (d, j 2 Hz); 6.85 1H (d, J = 8 Hz); 7.59 1H (d, d J = 2, 8 Hz); and 7.69 1H (d, J = 2 Hz). Compound 2, a yellowish brown solid, UV (MEOH), 256, 360 nm. 1H-NMR (CD3OD) ð (ppm): 6.2 1H (d, J = 2 Hz), 6.4 1H, d, J = 2 Hz), 6.92 (2H, d, J = 8 Hz), 7.92 2H, d (J = 8 Hz), ESI-MS M/Z (286) M+.
Column chromatography of the n-butanol-soluble part of the crude ethanolic extract and EAF of the stem-bark of CL and subsequent purification on Sephadex LH-20 afforded compound 1, a yellow solid. UV spectrum gave two λmax at 256 and 354 nm typical of bands 1 and 2 of a flavonoid nucleus. The proton NMR spectra revealed a 5, 7-dihydroxylated substituted pattern of a flavonoid, with two meta-coupled protons at ð 6.2 and 6.4 ppm and a 3′,4′ dihydroxylated pattern for ring B with an ABX aromatic system at ð 6.85, 7.59, and 7.69 ppm pointing toward quercetin. Compound 1 was in agreement with quercetin as reported in the literature. [32,33]
Compound 2 [Figure 1] is a yellowish brown solid isolated from the ethyl acetate-soluble part of the ethanol extract of the stem-bark of CL. The UV absorption maxima gave λmax at 256 and 360 nm, typical of a flavonoid nucleus. The proton NMR spectra displayed the characteristic signals of the kaempferol nucleus. [33] This substantiated by two doublets ðH 6.2 and 6.40 ppm (J = 2 Hz) and a pair of A2B2 aromatic proton system at ðH 6.90 and 7.90 ppm (J = 8 Hz). The LC-MS gave a chromatographic retention time of 22.6 min, while the electrospray ionization-MS in both positive and negative modes gave peaks at M/Z (287) and M/Z (285), which depict a molecular mass of M/Z (286) translating to a molecular formula C15H1006 which points to kaempferol. Compound 2 was found to be kaempferol by comparison of its NMR and MS with literature. [33]
Anti-inflammatory effects
The ESE of CL at a dose of 43.3 mg/kg demonstrate a significant anti-inflammatory activity (P < 0.05–0.001) when compared to control [Table 2], with 21.23% reduction of edema at the 3rd h [Figure 2]. The 86.6 and 173.2 mg/kg doses did not express any significant reduction of edema. This may be due to partial agonist activity. However, scrutinizing the anti-inflammatory activity of the extract by fractionation into four fractions revealed that the EAF (86.6 mg/kg) has the highest anti-inflammatory activity producing 89.0% reduction of edema at 3rd h. This was more potent than the pure drug, acetyl salicylic acid (100 mg/kg) which produces 65.8% reduction of edema. The order of anti-inflammatory activity of the fractions in relation to the crude extract is as follows: EAF > CHF > MTF > n-HF > ESE.
| Treatments(Dose)mg/kg | Total increase in Paw Volume (Mean±SEM) | Percent inhibition(at 3rdhr) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 hr | 2 hr | 3hr | 4 hr | 5 hr | 6 hr | ||
| Control | 4.68±0.06 | 5.87±0.08 | 5.68±0.09 | 6.14±0.15 | 6.01±0.13 | 5.84±0.16 | 5.78±0.10 | 0 |
| ESE 43.3 | 4.00c±0.08 | 5.22b±0.13 | 5.18c±0.07 | 5.15c±0.05 | 4.91c±0.19 | 5.33a±0.11 | 5.57ns±0.11 | 21.23 |
| ESE 86.6 | 4.12b±0.17 | 5.37a±0.12 | 6.00ns±0.09 | 6.32ns±0.12 | 6.37ns±0.14 | 6.19ns±0.19 | 6.69c±0.29 | −50.68 |
| ESE 173.2 | 4.16b±0.07 | 5.53ns±0.13 | 5.84ns±0.08 | 6.16ns±0.09 | 6.16ns±0.06 | 6.08ns±0.15 | 6.48b±0.16 | −36.99 |
| MTF 86.6 | 4.47ns±0.14 | 5.75ns±0.21 | 5.62ns±0.14 | 5.42c±0.10 | 5.38b±0.11 | 5.29a±0.11 | 5.24ns±0.14 | 45.09 |
| CHF 86.6 | 4.72ns±0.03 | 5.64ns±0.08 | 5.66ns±0.04 | 5.46c±0.05 | 5.45b±0.05 | 5.35a±0.06 | 5.29ns±0.04 | 45.59 |
| EAF 86.6 | 4.48ns±0.06 | 5.00c±0.05 | 4.77c±0.08 | 4.63c±0.09 | 4.68c±0.11 | 4.65c±0.07 | 4.64c±0.06 | 88.97 |
| n-HF 86.6 | 4.69ns±0.05 | 5.63ns±0.06 | 5.60ns±0.04 | 5.56c±0.04 | 5.52a±0.04 | 5.44ns±0.04 | 5.39ns±0.05 | 36.03 |
| MTF 173.2 | 4.72ns±0.06 | 5.78ns±0.04 | 5.61ns±0.02 | 5.51c±0.04 | 5.38b±0.03 | 5.22b±0.01 | 5.13a±0.03 | 41.91 |
| CHF 173.2 | 4.75ns±0.09 | 5.43ns±0.07 | 5.59ns±0.06 | 5.57c±0.06 | 5.51a±0.05 | 5.48ns±0.05 | 5.47ns±0.07 | 39.71 |
| EAF 173.2 | 4.18c±0.06 | 5.19c±0.05 | 5.10c±0.05 | 4.97c±0.04 | 4.90c±0.08 | 4.85c±0.03 | 4.91c±0.05 | 41.91 |
| n-HF 173.2 | 4.33ns±0.07 | 5.44ns±0.06 | 5.47ns±0.05 | 5.41c±0.05 | 5.39b±0.04 | 5.36a±0.04 | 5.35ns±0.05 | 20.59 |
| ASA 100 | 4.30ns±0.05 | 5.08c±0.03 | 4.89c±0.04 | 4.80c±0.05 | 4.71c±0.06 | 4.65c±0.05 | 4.70c±0.06 | 65.75 |
Significance relative to control: aP<0.05; bP<0.01; cP<0.001; values represent mean±SEM (n=6). ESE: Ethanolic stem-bark extract, MTF: Methanol Fraction of stem-bark at stated doses, CHF: Chloroform fraction, n-HF: n-Hexane Fraction, EAF: Ethylacetate Fraction, ASA: Acetylsalicylic acid (Aspirin)
Table 2: Effects of ethanolic stem-bark fractions of C. lutea on caragenin induced-paw oedema
However, in the egg albumin model, the lower dose of the crude extract of CL (43.3 mg/kg) demonstrated potent anti-inflammatory activity [Table 3] with a comparable reduction of paw edema when compared to the pure drug, aspirin (100 mg/kg). At 3rd h [Figure 3], the percentage reduction of paw edema was 74.0% compared to 73.0% elicited by 100 mg/kg aspirin, therefore proving demonstrable anti-inflammatory efficacy of CL than aspirin when compared to the control. Two doses of EAF (86.6 and 173.2 mg/kg) of all the fractions demonstrated a significant inhibition of paw edema (P < 0.01–0.001) compared to control. The degree of paw edema reduction was dose-dependent. However, the percentage reduction of edema by the two doses of CHF of 86.6 and 173.2 mg/kg are 53.4% and 26.0%, respectively. The crude ESE of CL (43.3 mg/kg) was more active in paw edema reduction than the median and high doses of the four fractions from the crude extract. The result indicates that the hydro ESE of CL commonly consumed by local people could have comparable anti-inflammatory effects to aspirin. In the capsaicin model of inflammation [Table 4], the result of the crude ESE of CL was very discriminatory between 43.3 and 86.6 mg/kg of the extract where the percentage reduction of edema at 3rd h increases from 7.6% to 25.8%. However, this effect was abolished at the high dose of the extract. The highest dose of n-HF (173.2 mg/kg) produced 42.0% reduction of edema compared to 51.0% by aspirin (100 mg/kg). The n-hexane extract and fractions and pure drug (aspirin) at all the doses evaluated in the capsaicin anti-inflammatory model were not significant (P > 0) when compared to control.
| Treatments(Dose)mg/kg | Total increase in Paw Volume (Mean±SEM) | Percent minhibition(at 3rdhr) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 hr | 2 hr | 3hr | 4 hr | 5 hr | 6 hr | ||
| Control | 4.90±0.32 | 5.59±0.09 | 5.75±0.25 | 5.67±0.18 | 5.63±0.14 | 5.49±0.16 | 5.33±0.12 | 0 |
| ESE 43.3 | 5.30ns±0 0.13 | 6.50c±0.24 | 6.00ns±0.32 | 5.50ns±0.21 | 5.36ns±0.16 | 5.18ns±0.20 | 5.19ns±0.14 | 74.03 |
| ESE 86.6 | 4.43ns±0.13 | 6.88c±0.18 | 6.44ns±0.12 | 5.97ns±0.19 | 5.54ns±0.08 | 5.25ns±0.11 | 5.11ns±0.15 | −100 |
| ESE 173.2 | 4.52ns±0.08 | 6.22a±0.24 | 5.65ns±0.19 | 5.27ns±0.09 | 5.01b±0.1 | 4.94a±0.06 | 4.64c±0.11 | 2.30 |
| MTF 86.6 | 4.91ns±0.03 | 6.54c±0.09 | 6.18ns±0.06 | 6.17ns±0.05 | 5.87ns±0.06 | 5.44ns±0.09 | 5.29ns±0.06 | −63.64 |
| CHF 86.6 | 5.23ns±0.10 | 6.08ns±0.10 | 5.74ns±0.15 | 5.59ns±0.16 | 5.48ns±0.17 | 5.39ns±0.17 | 5.35ns±0.17 | 53.25 |
| EAF 86.6 | 4.31a±0.02 | 4.89a±0.05 | 4.87b±0.09 | 4.80c±0.09 | 4.64c±0.07 | 4.70c±0.07 | 4.66b±0.07 | 36.36 |
| n-HF 86.6 | 4.66ns±0.05 | 6.12ns±0.10 | 5.70ns±0.03 | 5.51ns±0.03 | 5.33ns±0.02 | 5.18ns±0.04 | 5.10ns±0.04 | −10.39 |
| MTF 173.2 | 4.69ns±0.10 | 6.17ns±0.09 | 5.77ns±0.12 | 5.57ns±0.06 | 5.37ns±0.07 | 5.01ns±0.06 | 4.96ns±0.08 | −14.29 |
| CHF 173.2 | 4.91ns±0.02 | 6.27a±0.05 | 6.04ns±0.06 | 5.48ns±0.09 | 5.30ns±0.07 | 5.21ns±0.08 | 5.03ns±0.11 | 25.97 |
| EAF 173.2 | 4.39ns±0.09 | 5.59ns±0.02 | 4.94a±0.05 | 4.93b±0.04 | 4.80c±0.06 | 4.81b±0.08 | 4.73b±0.08 | 29.87 |
| n-HF 173.2 | 4.64ns±0.02 | 5.78ns±0.02 | 5.65ns±0.02 | 5.56ns±0.01 | 5.45ns±0.02 | 5.31ns±0.01 | 5.19ns±0.02 | −19.48 |
| ASA 100 | 4.49ns±0.07 | 5.34ns±0.12 | 4.91b±0.03 | 4.70c±0.05 | 4.61c±0.06 | 4.65c±0.06 | 4.65c±0.05 | 72.72 |
Significance relative to control:aP<0.05; bP<0.01; cP<0.001; values represent mean±SEM (n=6). MTF: Methanol Fraction of stem-bark at stated doses, CHF: Chloroform fraction, n-HF: n-Hexane Fraction, EAF: Ethyl acetate Fraction, ASA: Acetylsalicylic acid (Aspirin)
Table 3: Effects of C. lutea ethanolic stem-bark extract and fractions on egg albumin-induced rat paw oedema
| Treatments(Dose)mg/kg | % Increase in Paw Volume (Mean±SEM) | Percent inhibition(at 3rdhr) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 hr | 2hr | 3hr | 4 hr | 5 hr | 6 hr | ||
| Control | 4.70±0.04 | 5.11±0.13 | 5.30±0.03 | 5.36±0.05 | 5.33±0.05 | 5.44±0.04 | 5.50±0.04 | 0 |
| ESE 43.3 | 4.18b±0.11 | 5.08ns±0.19 | 4.93ns±0.05 | 4.79ns±0.15 | 4.96 ns±0.14 | 5.00ns±0.16 | 5.00 ns±0.10 | 7.58 |
| ESE 86.6 | 4.46ns±0.09 | 5.14ns±0.10 | 4.96ns±0.05 | 4.95 ns±0.04 | 5.03 ns±0.09 | 5.03 ns±0.07 | 5.01 ns±0.07 | 25.76 |
| ESE 173.2 | 4.34ns±0.05 | 5.21ns±0.17 | 4.86ns±0.08 | 5.01ns±0.15 | 5.14 ns±0.11 | 5.20 ns±0.09 | 5.04 ns±0.13 | −1.52 |
| MTF 86.6 | 4.56ns±0.09 | 5.90a±0.17 | 5.77ns±0.20 | 5.87ns±0.10 | 5.71ns±0.06 | 5.65ns±0.06 | 5.49ns±0.11 | −98.48 |
| CHF 86.6 | 4.57ns±0.15 | 5.75ns±0.20 | 5.55ns±0.21 | 5.40ns±0.19 | 5.28ns±0.15 | 5.25ns±0.11 | 5.15ns±0.10 | −40.91 |
| EAF 86.6 | 4.69ns±0.05 | 5.41ns±0.05 | 5.27ns±0.03 | 5.19ns±0.03 | 5.12ns±0.03 | 5.24ns±0.03 | 5.24ns±0.02 | 24.24 |
| n-HF 86.6 | 4.67ns±0.10 | 5.52ns±0.13 | 5.45ns±0.14 | 5.42ns±0.14 | 5.47ns±0.17 | 5.28ns±0.18 | 5.25ns±0.13 | −13.64 |
| MTF 173.2 | 4.51ns±0.07 | 5.63ns±0.22 | 5.45ns±0.15 | 5.27ns±0.14 | 5.19ns±0.08 | 5.15ns±0.10 | 5.08ns±0.13 | −15.15 |
| CHF 173.2 | 4.35ns±0.07 | 5.38ns±0.17 | 5.38ns±0.17 | 5.41ns±0.21 | 5.27ns±0.21 | 5.39ns±0.17 | 5.18ns±0.15 | −60.61 |
| EAF 173.2 | 4.70ns±0.07 | 5.33ns±0.07 | 5.26ns±0.09 | 5.25ns±0.07 | 5.35ns±0.03 | 5.40ns±0.08 | 5.42ns±0.04 | 16.67 |
| n-HF 173.2 | 4.63ns±0.10 | 5.72ns±0.11 | 5.40ns±0.13 | 5.21ns±0.12 | 5.37ns±0.20 | 5.37ns±0.19 | 5.23ns±0.16 | 42.0 |
| ASA 100 | 4.72ns±0.06 | 5.49ns±0.17 | 5.17ns±0.16 | 5.12ns±0.10 | 5.26ns±0.06 | 5.25ns±0.05 | 5.26ns±0.04 | 51.0 |
Significance relative to control: aP<0.05; bP<0.01; cP<0.001; values represent mean±SEM (n=6). MTF: Methanol Fraction of stem-bark at stated doses, CHF: Chloroform fraction, n-HF: n-Hexane Fraction, EAF: Ethyl acetate Fraction, ASA: Acetylsalicylic acid (Aspirin)
Table 4: Effects C. lutea ethanolic stem-bark fractions on capsiacin-induced paw oedema
Antinociceptive effects
Acetic acid-induced writhing test
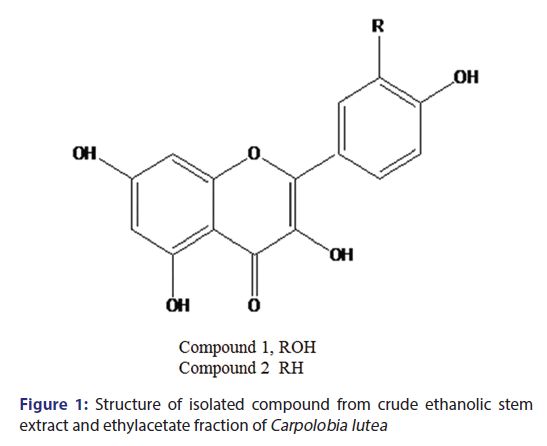
The intraperitoneal injection of acetic acid (6.0%) caused a powerful nociceptive response in the control group, with CL ESE significantly (P < 0.5–0.001) reduced writhing reflex induced by acetic acid [Figure 4]. The protective effects were observed to be 58.26%, 70.94%, and 64.96% inhibitions for the 43.3, 86.6, and 172.3 mg/kg ESE, respectively, while paracetamol (130 mg/kg) produced 85.33% inhibition of nociception. Pretreatment of the mice with ESE (86.6 mg/kg) followed by 15 min later with paracetamol (130 mg/kg) produced 25.50% inhibition of writhing reflex. Antinociceptive effect was expressed as PAA as shown in Figure 4.
Formalin test
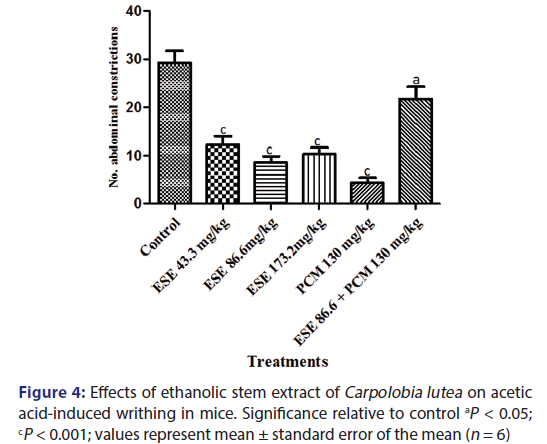
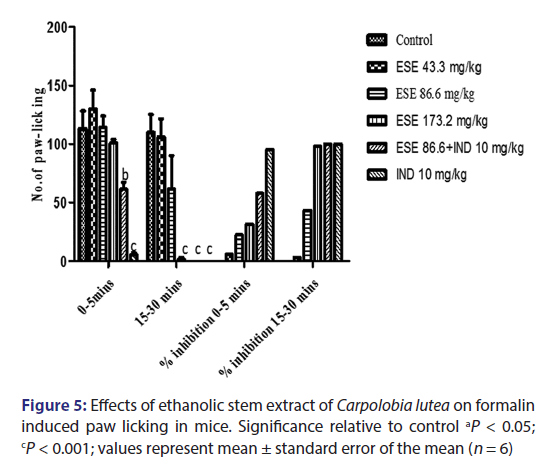
Injection of formalin into the hind paw of the mice produced a marked biphasic response [Figure 5]. The first phase occurs 5 min after injection and the second phase occurs 15–30 min after the injection of formalin. The mean licking time of the first and second phase reaction time after drug administration reduced in a dose-dependent manner. Pretreatment with CL ESE (43.3 and 86.6 mg/kg) resulted in 6.10% and 22.50% inhibition of nociception for first-phase reaction time and 3.35% and 43.53% nociceptive index for second-phase reaction time which are not statistically significant (P > 0). The high dose of ESE (173.2 mg/kg) did not significantly inhibit nociception in the first phase but does in the second phase (P < 0.001) with nociceptive indices of 31.53% and 98.03%, respectively. Administration of ESE 86.6 mg/kg and indomethacin (10 mg/kg) 15 min later produces 58.08% inhibition of paw licking in the first phase (P < 0.01) and 100% inhibition in the second phase (P < 0.001) when compared to the control group. The administration of pure drug indomethacin (10 mg/kg) alone produces 95.2% inhibition of the first phase and 100% inhibition of the second phase (P < 0.001) when compared to the control.
Hot plate
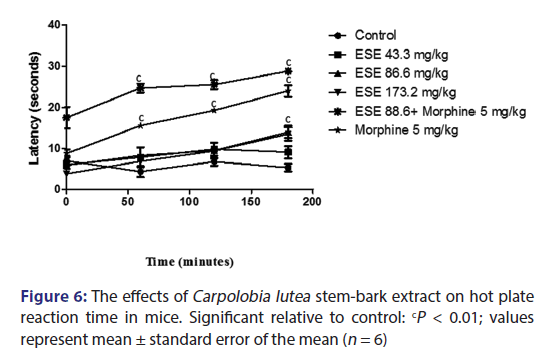
The results from the hot plate test indicate that treatment with 43.3, 86.6, and 173.2 mg/kg of the ESE of CL administered intraperitoneally did not give any statistically significant inhibition of nociception induced by hot plate when compared with the control group. However, the pure drug morphine (5 mg/kg) alone; and ESE (86.6 mg/kg) plus morphine (5 mg/kg) were statistically significant (P > 0.001) when compared to control as shown in Figure 6.
Discussion
The study indicates that ESE exhibited significant anti-inflammatory and antinociceptive effects which might in part be due to bioactive molecules in the plant extract and fractions. Subcutaneous injection of carrageenin into the rat paw produces inflammation resulting from plasma extravasation, increased tissue water, and plasma protein exudation along with neutrophil extravasations, all due to the metabolism of arachidonic acid. [34] The first phase begins immediately after the injection of carrageenan and diminishes in 3 h. The second phase begins at the end of the first phase and remains through 3rd h up to 5th h. The continuity between the two phases is provided by kinins. [35] COX-2 is an inducible isoform found in activated inflammatory cells that generates prostanoid mediators of inflammation. [36] Taking together, the anti-inflammatory mechanism of CL stem-bark fractions may in part be due to either the inhibition of histamine, serotonin, kinin, and PG biosynthesis enzymes responsible for plateau and second accelerating phases of inflammation, increased vascular permeability, or by any other mechanism. The result indicates that the fractions were active in both the early and late phases of carrageenin-induced hind paw inflammation in rats. Considering the doses of fraction utilized in this study, the high dose of n-HF (173.2 mg/kg) significantly reduced the paw volume from 1 to 5 h with the maximal effects observed at the 2nd and 3rd h, respectively. The lower dose of EAF (86.6 mg/kg) used was found to be more active than the highest dose of EAF (173.2 mg/kg) and with a maximal inhibition greater than aspirin, the standard drug. This result further suggests that CL stem-bark fractions could possibly exert its effect by inhibiting the COX pathway. Aspirin is a NSAID which acts by the irreversible inactivation of both COX-1 and COX-2. [37]
Similarly, in the egg albumin-induced edema, all the rats pretreated with the fractions significantly (P < 0.05–0.01) showed reduced paw edema relative to control. In this model, edema peaked at 30 min to 1 h and gradually declined at 3rd h and up to 5 and 6 h, respectively. The EAF of 86.6 mg/kg gave the maximal inhibition compared with all the fractions relative to control. Egg albumin has been known to cause inflammation by inducing the release of two inflammatory mediators which are basically histamine and serotonin, [38] which the fractions inhibited to reduce inflammation.
Phytochemicals such as alkaloids, saponins, cardiac glycosides, anthraquinone, and tannins which were to a large extent similar in distribution in both extract and fractions except for few increase of anthraquinone in the chloroform and EAFs, all have been implicated to possess anti-inflammatory and antinociceptive effects. [10] Phenolic compounds have significant pharmacological value as anti-inflammatory agents. [11] Flavonoids are reported to provoke a decrease in the expression of inflammatory signaling molecules such as inhibition of inducible nitric oxide synthase (iNOS) expression, inhibition of COX-2 expression, inhibition of leukocyte activation, and inhibition of platelet aggregation and direct vasodilatory action; [12] flavonoids and condensed tannins are known to inhibit some molecular targets of pro-inflammatory mediators in inflammatory responses. [13-15] The mechanisms underlying the anti-inflammatory effect of tannins include the scavenging of radicals and inhibition of the expression of inflammatory mediators, such as some cytokines, inducible nitric-oxide synthase, and COX-2. [16] Some alkaloids such as isoquinoline, indole, and diterpene are known to have good anti-inflammatory activity. [17] Anti-inflammatory activities of some saponin derivatives such as triterpenoids saponins have been reported. [10] The two flavonoid compounds, quercetin and kaempferol, isolated from the extract and fraction, respectively, are reported to exhibit marked anti-inflammatory and antinociceptive effects. Kaempferol and quercetin are reported to inhibit iNOS protein and mRNA expression and also prevent NO production in a dose-dependent manner; they are useful inhibitors of nuclear factor-kappa B and the signal transducer and activator of transcription 1, another important transcription factor for iNOS. [39] Kaempferol shows anti-atherosclerotic effect by modulating the gene and protein expression of inflammatory molecules. [40] Both flavonoids significantly inhibited mRNA level of iNOS, COX-2, and C-reactive protein (CRP). [41] Kaempferol produced a significant concentration-dependent decrease of iNOS, COX-2, and CRP level at all concentrations, but the percentage of inhibition induced by quercetin was reduced at high concentrations. Similarly, as the dose of ESE increases, it did not exhibit significantly higher anti-inflammatory or antinociceptive effects.
The extract inhibited the acetic acid-induced abdominal constriction response in a dose-dependent manner. The abdominal constriction is related to the sensitization of nociceptive receptors to prostaglandins (PGs). It is therefore possible that ESE exerts an antinociceptive effect, probably by inhibiting synthesis or action of PGs. This is corroborated by the marked inhibitory effects of the low dose of ESE and the EAF on carrageenin-mediated inflammation. Acetic acid-induced pain due to capillary permeability is used in the assessment of antinociceptive activity in mice.
Acetic acid induces writing and produces algesia through the releases of endogenous mediators such as cytokines and eicosanoids with concomitant increase in peritoneal fluid levels of PG E2, which then stimulate the pain-sensitive nerve endings. [42] The administration of acetic acid (i.p.) irritates serous membranes and provokes a stereotypical behavior in mice characterized by abdominal contractions, movements of the whole body, twisting of dorso-abdominal muscles, and a reduction in motor activity and coordination. [43] The administration of the stem extract reduces abdominal constriction dose dependently and significantly for all doses (P < 0.001). The mechanism may be inhibition of release of proinflammatory mediators or direct blockade of receptors mediating their release or both in peripheral eicosanoid pathways.
The characterization and isolation of quercetin and kaempferol from EAF reported might in part be implicated in the observed pharmacological property. Chronic inflammation associated with different types of diseases, arthritis, allergies, atherosclerosis, and even cancer has been remedied recently by natural product-based drugs which are considered as the novel therapeutic strategy for the prevention and treatment of inflammatory diseases. Kaempferol and quercetin present in this natural products are widely reported and reviewed to ameliorate inflammation under both in vitro and in vivo conditions. [44,45]
Conculsion
The isolation of quercetin and kaemferol from CL extracts gave credence to its anti-inflammatory and antinociceptive effects. The high patronages of illicit gin soaked with CL stem-bark justify its use to alleviate inflammatory and algesic stimulus in ethnomedicine of the Ibibios in Akwa Ibom State.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Malik M, Parikh I, Vasquez JB, Smith C, Tai L, Bu G, et al. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol Neurodegener 2015;10:52.
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, et al. Neuroinflammation in Alzheimer’s disease and prion disease. Glia 2002;40:232-9.
- Mancuso C, Scapagini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, et al. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 2007;12:1107-23.
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol 2002;1:232-41.
- Kriz J. Inflammation in ischemic brain injury: Timing is important. Crit Rev Neurobiol 2006;18:145-57.
- Fawole OA, Ndhlala AR, Amoo SO, Finnie JF, Van Staden J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J Ethnopharmacol 2009;123:237-43.
- Abu-Rabia A. Urinary diseases and ethnobotany among pastoral nomads in the Middle East. J Ethnobiol Ethnomed 2005;1:4.
- Bouayed J. Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci 2010;6:13-8.
- Bouayed J, Bohn T. Exogenous antioxidants – Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 2010;3:228-37.
- Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol 2004;94:219-43.
- Bruneton J. Pharmacognosy, Phytochemistry of Medicinal Plants. France: Lavoisier; 1995. p. 265-380.
- Mladenka P, Zatloukalová L, Filipský T, Hrdina R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic Biol Med 2010;49:963-75.
- Weniger B, Vonthron-Sénécheau C, Kaiser M, Brun R, Anton R. Comparative antiplasmodial, leishmanicidal and antitrypanosomal activities of several biflavonoids. Phytomedicine 2006;13:176-80.
- Iwalewa EO, McGaw LJ, Naidoo V, Eloff JN. Inflammation: The foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr J Biotechnol 2007;6:2868-85.
- Wang XH, Xu B, Liu JT, Cui JR. Effect of beta-escin sodium on endothelial cells proliferation, migration and apoptosis. Vascul Pharmacol 2008;49:158-65.
- Erdèlyi K, Kiss A, Bakondi E, Bai P, Szabó C, Gergely P, et al. Gallotannin inhibits the expression of chemokines and inflammatory cytokines in A549 cells. Mol Pharmacol 2005;68:895-904.
- Barbosa-Filho JM, Piuvezam MR, Moura MD, Silva MS, Lima KV, Leitão da-Cunha EV, et al. Anti-inflammatory activity of alkaloids: A twenty-century review. Rev Bras Farmacognosia 2006;16:109-39.
- Nwidu LL, Nwafor PA, Vilegas W. The aphrodisiac herb Carpolobia: A biopharmacological and phytochemical review. Pharmacogn Rev 2015;9:132-9.
- Nwidu LL, Nwafor PA. Gastroprotective effects of leaf extracts of Carpolobia lutea (Polygalaceae) G. Don. in rats. Afr J Biotechnol 2009;8:15-9.
- Nwidu LL, Nwafor PA, da Silva VC, Rodrigues CM, dos Santos LC, Vilegas W, et al. Anti-nociceptive effects of Carpolobia lutea G. Don (Polygalaceae) leaf fractions in animal models. Inflammopharmacology 2011;19:215-25.
- Nwidu LL, Ukiri OO, Rodrigues CM, Vilegas W. Antidiarrheal mechanism and ionic profile of Carpolobia lutea ethanolic stem-bark extract in rats. Afr J Tradit Complement Altern Med 2014;11:257-63.
- Nwidu LL, Nwafor PA, Vilegas W. Antimicrobial activity of Carpolobia lutea extracts and fractions. Afr J Tradit Complement Altern Med 2012;9:323-8.
- Nwidu LL, Nwafor PA. Anti-inflammatory and antipyretic effect of Carpolobia lutea Leaf extract in rodents. Int Res J Pharm 2012;3:154-60.
- Nwidu LL, Nwafor PA, Vilegas W. Neuropharmacological screening and isolation of cinnamoyl and coumaroyl-glucosides from leaf fraction of Carpolobia lute G. Don (Polygalaceae). Indian J Nov Drug Deliv 2012;4:28-37.
- Nwidu LL, Nwafor PA, Vilegas W. Antiulcer effect of ethyl acetate extract of Carpolobia lutea Leaf. J Appl Pharm Sci 2012;2:233-42.
- Nwidu LL. Pharmacological Characterization of Antiulcer Principles in C. lutea Leaf. A Ph.D Thesis of the Faculty of Pharmacy, University of Uyo; 2010. p. 211-2.
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983;54:275-87. 28.
- Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544-7.
- Arya S, Kumar VL. Antiinflammatory efficacy of extracts of latex of Calotropis procera against different mediators of inflammation. Res Commun 2005;4:228-32.
- Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol 2007;109:219-25.
- Iwueke AV, Nwodo OF, Okoli CO. Evaluation of the anti-inflammatory and analgesic activities of Vitex doniana leaves. Afr J Biotechnol 2006;5:1929-35.
- Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York: A Springer-Verlag Publication; 1970. p. 65-73, 126-34.
- Fossen T, Oyvind M. Spectroscopic technique applied to flavonoids. In: Anderson M, Markham KR, editors. Flavonoids, Chemistry, Biochemistry and Applications. New York: A Taylor Publication; 2006. p. 53-5.
- Harbone JB, Mabry TJ, Mabry H, editors. The Flavonoids. Vol. 1. New York: Academic Press; 1975.
- Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 1971;104:15-29.
- Sawatzky DA, Megson IL, Rossi AG. Sildenafil offers protection against NSAID-induced gastric injury. Br J Pharmacol 2005;146:477-8.
- Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Edinburg: Churchill Livingstone; 2003. p. 217-41.
- Akah PA, Nwambie AI. Evaluation of Nigerian traditional medicines: 1. Plants used for rheumatic (inflammatory) disorders. J Ethnopharmacol 1994;42:179-82.
- Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007;2007:45673.
- Kong L, Luo C, Li X, Zhou Y, He H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis 2013;12:115.
- García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol 2007;557:221-9.
- Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic Alalgesics. Br J Pharmacol Chemother 1964;22:246-53.
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001;53:597-652.
- Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, et al. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res 2015;99:1-10.
- Kelly GS. Quercetin. Monograph. Altern Med Rev 2011;16:172-94.