Anti-diabetic Activity of Compound "2-[(trimethylsilyl) oxy] - Methyl Ester (cas) Methyl-o-trim-ethyl-silylsalicylate" Isolated from Pericampylus glaucus (Lam) Merr in STZ-Induced Diabetic Rats
- *Corresponding Author:
- Muhammad Kifayat Ullah
PhD Research Fellow, Department of Pharmacology and Toxicology
Faculty of Pharmacy, Lincoln University College, No. 2, Jalan Stadium
SS 7/15, 47301 Petaling Jaya, Selangor Darul Ehsan, Malaysia.
E-mail: kifayatpharma86@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background and Objectives: Diabetes mellitus is a chronic metabolic disorder, which is characterized by alterations in carbohydrates, protein and fats metabolism and have been connected to all age’s people worldwide. Thus, the aim of present research work was to isolate and determine the anti-diabetic activity of compound from the plant P. glaucus (Lam) Merr in STZ-induced diabetic rats. Materials and Methods: The isolation and identification of compound was determined with the help of column chromatography followed by TLC, GCMS and 1HNMR. The anti-diabetic profile of the isolated compound was investigated in STZ- induced diabetic rats at single dose 50 mg/kg (b.wt). The glibenclamide at a dose of 20 mg/kg (b.wt), p. o) was used as a standard. Results and Discussion: The compound benzoic 2-[(trimethylsilyl) oxy]-, methyl ester (cas) methyl o-trim ethyl-silylsalicylate was isolated from active fractionation with the help of column chromatography followed by TLC, GCMS and 1HNMR. The RF value determined on TLC plate was 0.33. The M.wt of isolated compound was 308; H NMR (399.6 MHz); retention time 22.26; mz 209; % area 7.43 and area was 2110418.00. The molecular formula was C21H22O2. The isolated compound significantly (P<0.001) reduced blood sugar level in STZ-induced diabetic rats at single dose 50 mg/kg (b.w) respectively as matched to untreated diabetic control group. The attenuation in blood sugar level with isolated compound was 15.10 ± 0.09 mmol/L at 6 hrs, 13.70 ± 0.09 mmol/L at 12 hrs and 11.15 ± 0.25 mmol/L at 24 hours as matched to untreated diabetic control group. Conclusions: The conclusions of this in vivo experimental study indicate that the compound isolated from P. glaucus possesses significant hypoglycemic properties; and thus provides the justification of P. glaucus in the remedy and as well as in the management of diabetes mellitus.
Key words
Diabetes mellitus, isolated compound, P. glaucus; TLC, GCMS, 1HNMR, 2-[(trimethylsilyl) oxy]-, methyl ester (cas) methyl o-trim ethyl-silylsalicylate, STZ, Sprague-Dawley rats
Introduction
Diabetes mellitus is a persistent metabolic disorder, which is characterized by variations in the metabolism of carbohydrates, protein and fats and result rise in blood sugar levels.[1] According to IDF worldwide, 382 million of population are suffering from diabetes and will raise to 592 million in 2035.[2] In Malaysia, prevalence of diabetic patient almost become double within an interval of twenty years increased from 8.3% that was in 1996[3] to 14.9% that was 2006[4] and 20.8% in 2011.[5] The projection of diabetic patients in Malaysia for 2030 was 2.48 million according to IDF.[6] But, the latest survey from Ministry of Health Malaysia, confirmed that 3.3 million of Malaysian experiencing diabetes, which is quite more as it was estimated for 2030.[7] Allopathic drugs for diabetes mellitus have been constrained due associated side effects.[8] The ethno-botanical reports indicates 25, 0000 to 50, 0000 species of plant are present worldwide and about 800 of natural products may have anti-hyperglycemic prospects.[9,10] The plant Pericampylus glaucus Lam Merr belongs to the family of Menispermaceae and commonly found throughout Malaysia, China, Japan, Bangladesh, Indonesia, Myanmar, Taiwan, Nepal, Philippine and Vietnam.[11] The various parts of the plant are being effectively used traditionally for remedies of various human disorders.[1] The leaves along with roots are taken as oral decoction to treat high level of sugar in the blood and hypercholesterolemia in Malaysia.[12] In Nepal, the plant is used for the treatment of constipation, urination pain (dysuria) and high blood pressure.[13] Previously, the plant was reported for having acceptable results against excessive free radicals, cancer, AIDS, HBV and HCV properties.[11,14]
The ethanolic plant extract of Pericampylus glaucus Lam Merr have been reported for having in-vitro antioxidant activities at different concentrations by DPPH and reducing powder assays.[11] The alkaloids including Periglaucine A, B, C and D along with three well known alkaloids, oxotetrahydropalmatine, norruffscines and oxocanadine were isolated from Pericampylus glaucus plant and have proved experimentally in laboratory for having significant effects against Hepatitis B, Hepatitis C and HIV- 1 virus.[14] The triterpenes such as hopenone, hopenol, 22-hydroxyhopan-3-one, erythrodiol 3-palmitateand 5β, and 24-cyclofriedelan-3-onerespectively were isolated from plant Pericampylus glaucus and have been investigated for anti-cancer activity.[15] The activity of plant against cancer was assayed by colorimetric technique to determine the number of living cell present. The compound erythrodiol 3-palmitateand 5β was noted to have more positive result in inhibition the production of K562 cells. [15] It was also reported about the isolation of six crystalline compounds such as melissic acid, epifriedelinol, palmatic acid, bututic acid stearic acid and daucosterol from the rhizome of plant Pericampylus glaucus. [16] Among six crystalline compounds two compounds; palmatic acid and daucosterol isolated from other medicinal plants have been proved for having significant antihyperlipidemic and antihyperglycemic effects in experimental animal model.[17,18] The preliminary plant extracts of Pericampylus glaucus have also confirmed for possessing anti-malarial activity against plasmodium species; falciparum and was helpful in reducing and diminishing of the hematological changes that was caused by Plasmodium falciparum.[19] A significant result was observed against the malaria parasite. The leaves of ethanolic plant extract of Pericampylus glaucus was investigated scientifically at three doses 400, 600 and 800 mg/kg (b.wt) for having anti-diabetic activities in STZ-induced high fats diets and was scientifically proved for having positive significant effect against hyperglycemia in diabetic induced animal’s model.[1] Recently, a compound “Periglaucine A”, which was isolated from plant Pericampylus glaucus and have been reported in vitro nasopharyngeal carcinoma and anti-inflammatory.[20] Therefore, the present study was aimed to isolate and determine the anti-diabetic activity of compound from Pericampylus glaucus Lam Merr in STZinduced diabetic rats.
Materials and Methods
Drugs and chemicals
Streptozotocin and glibenclamide were purchased from Sigma-Aldrich Bio Syn Tech Malaysia group. The chemicals used were analytical grade, astral laboratory chemicals (Mangrove Lane Taren Point NSW 2229 Australia), R/M chemical (Barotiwala, Himachal Pradesh 173201, India) and Sigma-Aldrich Co. The glucose assay kit used was Sigma Diagnostics, Inc.
Instruments
The instrument used for the present study was GCMS (GC model 2014), S/N E11484913004, P/N=221-70020-34, GC-2014 AFC 230 V, Shimadzu, UV (Model 1800) S/No=A11454908357), P/N=206-25400-38, Shimadzu and 1HNMR (JNM- ECZS series, FT-NMR, manufacturer (JEO USA).
Test animal
Adult Sprague-Dawley rats having weight of 90-110 g were used and were kept in animal holding facility centre, Department of Pharmacology, Lincoln University College, Malaysia. The experimental protocols used was approved by the Lincoln University College, Malaysia, Animal Ethics Committee (LUC-AEC), with reference LUC-AEC number PHARM/2013/MSM/02-August/11/September 2014-August 2016 after confirming that the proposed research work complies all with Malaysian regulation.
Collection and identification of plant material
The plant Pericampylus glaucus was collected from Negri Sembilan state of Malaysia in 2014 and was authenticated from Forest Research Institute Malaysia (FRIM); with specimen herbarium number (FRIM/394/490/5/18(118)).
Extraction of plant material
The leaves were dried in shade for 20 days and converted into coarse powder with the help of mechanical grinder. The powder (500 gm) of Pericampylus glaucus was extracted by hot extraction method using the soxlet apparatus at a temperature of 78°C using ethanol solvent. The extract was then concentrated under reduce pressure through rotary evaporator (N- 10000, Eyela, Japan) and was preserved in a desiccators for further pharmacological activities.
Preliminary phytochemical screening of plant extract
The presence of phytochemical in Pericampylus glaucus ethanolic extract was previously screened by various chemical identification tests.[11]
Fractionation of ethanolic extract for identification of active compound
The ethanolic extract of P. glaucus (20 gm) was subjected further for the process of fractionation and purification by the method as was used by researcher (Natarajan), with some alterations in procedure.[17] The ethanolic plant extract was combined with equivalent amount of 20 gm of silica gel and dried completely. The column was effectively eluted 1/3rd through different solvents system, started from n-hexane followed by chloroform, petroleum ether, ethyl acetate, acetone and ethanol in different concentration ratio like n-hexane (100%), n-hexanechloroform mixture (30:70), chloroform-petroleum ether mixture (30:70), petroleum ether and ethyl acetate mixture (70:30), ethyl acetate and acetone mixture (70:30) and finally 95% ethanol 100%.[21]
Identification of compound by TLC
The major fraction after elution with petroleum ether and ethyl acetate mixture (70:30) was collected and was further subjected for fractionation and purification with the help of column chromatography using solvent system ethanol: chloroform: (70:30). Only, one fraction was observed in column and was collected separately. The isolated fraction was validated by TLC.[22] Two droplets from sample was dotted on prepared TLC silica gel plates and immersed on close fitting thin layer chromatography chroma tank chamber that comprised mixture of chloroform and methanol and was stand for a period of 20 minutes. After 1 hr, plate was sprayed with sulphuric acid and methanol reagent and was observed under UV for the presence of compound once dried. The retention factor (RF) values were measured.
Identification of compound by GC-MS technique
The GC-MS analysis of petroleum ether and ethyl acetate fraction, collected through column chromatography, was carried out on a GC CLARUS 550 PerkinElmer system that was composed of a gas chromatograph connected to a mass spectrometer (GC-MS) apparatus providing the following specifications: The non-polar Elite-1 column which comprised dimethyl-poly-siloxane (100%) was fused to capillary column (Restek silica) (30 × 0.25 mm ID × 1EM df. The oven temperature was fixed from 110°C (isothermal for 2 min), with an increase of temperature 10°C/minutes, up-to 200°C, then increase temperature 5°C/min to temperature 280°C and ending with a 9 min isothermal at 280°C respectively. The mass spectrums were taken at 70 eV; with scan duration of 0.5 sec and the fragments were from 40 to 550 Daltons respectively and were searched on computer NIST MS data library for comparing the spectrum through GC-MS. The identification of compound existing in fraction of Pericampylus glaucus was achieved with the help of direct comparison of retention time and mass spectra with the spectra of known compounds using the record library combined with the NIST) library. The name, molecular formula, molecular weight and area under peak (percentage by peak area) of the component of the test materials (petroleum ether and ethyl acetate fraction of Pericampylus glaucus) were determined. The name, molecular weight, retention time, area and structure of the compound were determined GC-MS analysis.[23]
1HNMR analysis of compound
Hydrogen-NMR spectra of the fraction collected from plant Pericampylus glaucus was performed using Bruker Biospin Avance 400 MHz NMR spectrophotometer with a 5 mm broad inverse probe head, equipped with shielded z-gradient accessories. The hydrogen-NMR spectral analysis of the fraction was carried out using one-pulse sequence by dissolving the fraction collected from column chromatography in 500 μL of deuterated methanol in 5 mm NMR tubes. A re-usable, sealed capillary tube, which comprising a volume of 30 μL of 0.375% of tri-sodium phosphate in deuterium oxide was introduced into the NMR tube prior recording the spectrum. Tri-sodium phosphate performed as chemical shift standard as well as internal reference for the quantitative inferences.[24]
Acute toxicity study
The ethanolic extract of Pericampylus glaucus was investigated previously up-to dose of 4000 mg/kg (b.wt) orally and was confirmed that lethal dose might be more than 4000 mg/kg (b.wt).[25] A single dose of 50 mg/kg (b.wt) of compound isolated was chosen for present research.
Induction of Diabetes mellitus
The diabetes was induced in animal’s model by low single dose of STZ 50 mg/kg b.wt (IP) that was dissolved in fresh prepared 0.01M citrate buffer having pH was 4.5 on pH meter. The animals having the blood sugar levels more 14 mmol/L were selected for experiment after 72 hrs of injection of STZ.[1]
Effect of compound on blood glucose in STZinduced diabetic rats
The effect of compound on blood glucose in STZ-induced diabetic rats was determined by the method as was used by (Kumar et al.) by making some changes in procedure.[26] The normal and STZ-induced diabetic rats were divided into four groups with four animals in each group. The 1st group received only with normal saline. The 2nd group was kept as untreated diabetic group. Group III and IV diabetic animals were treated with isolated compound at dose 50 mg/kg (b.wt) and glibenclamide at dose 20 mg/kg (b.wt). The animals were kept on fasting for an overnight (12-14 hrs) before administration of single dose of isolated compound and standard drug orally. The blood samples were collected from the tail vein at 0, 3, 6, 9, 12 and 24 hrs after the administration of isolated compound and standard and the blood glucose levels were determined by using glucose oxidize standard kits.[27]
Statistical Analysis
Statistical analysis was performed as mean of variance ± SEM (N=4) followed by ANOVA test using Graph Pad Prism and for multiple comparison test among the groups, Bonferroni test was performed. A probability level of P<0.05 was accepted statistically.
Results
Identification of the compound by TLC
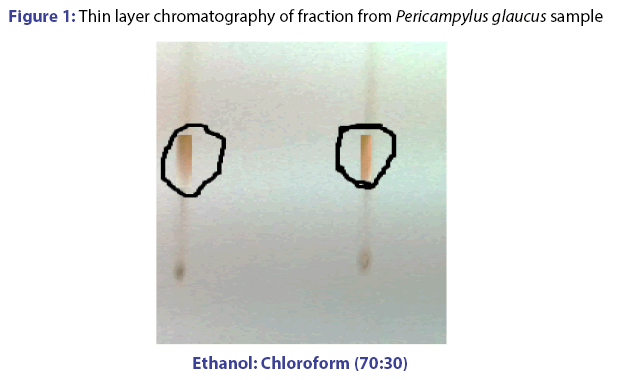
Base on column chromatography, using petroleum ether: ethyl acetate mixture, a brown paste like compound was collected after passing again through ethanol and chloroform in column chromatography. TLC plate strongly indicates the presence of single compound in fraction, which was placed on prepared TLC plate and was found brown chocolate color on UV spectrophotometer. The retention factor (RF) values measured for same spot and same color on thin layer chromatography (TLC) plate was 0.33. The result is presented in Figure 1.
Identification of compound by GC-MS technique
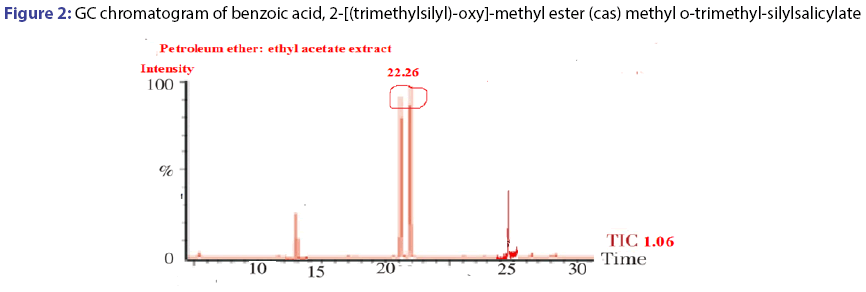
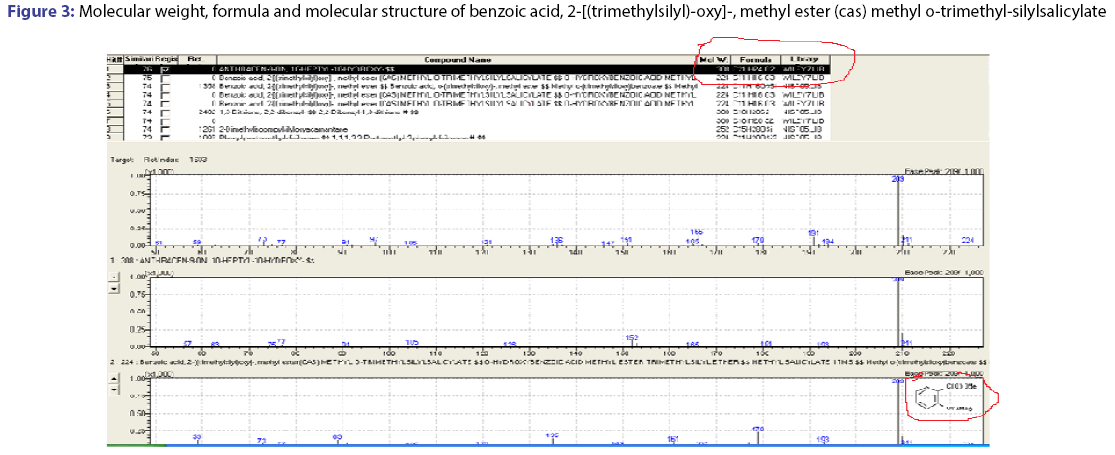
The GC-MS spectral data of petroleum ether and ethyl acetate fraction, collected through column chromatography confirmed the presence of single compound belongs to the benzoic acid family. The IUPAC name of the compound identified through GC-MS was 2-[(trimethylsilyl)- oxy]-methyl ester (cas) methyl-o-trimethyl silylsalicylate. The yield of isolated compound in P. glaucus leaves power was 0.65%. The compound was identified based on the following evidence: molecular weight 308; retention time 22.26; mz 209; % area 7.43 and the area were 2110418.00. The molecular formula of 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethyl-silylsalicylate was C21H22O2. The molecular structure, mass spectra and GC chromatogram of the compound are shown in Table 1; Figures 2 and 3.
| Sample | Name of compound | M.F | M.wt | RT | % area |
|---|---|---|---|---|---|
| 1 | 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethyl-silylsalicylate | C21H22O2 | 308 | 22.26 | 7.43 |
Table 1: GC-Ms analysis of the compound isolated from Pericampylus glaucus
1H NMR Analysis
Hydrogen-1HNMR-based analysis was sufficient to generate data from a sample within a relatively short time. Hydrogen-1HNMR and C13 NMR spectra of petroleum ether and ethyl acetate fraction revealed a single signal as graphically presented in Figure 4 respectively. The compound identified in Pericampylus glaucus fraction was independently characterized by means of their particular chemical shifts principles as revealed in the spectra below. The 1H NMR was 399.6 MHz.
Effect of isolated compound on blood glucose in STZ-induced diabetic rats
The effect of isolated compound and standard drug on fasting blood glucose levels in STZ-induced diabetic rats are presented in Table 2. The result showed that the blood glucose levels of all treated groups especially II that was kept as untreated diabetic group was significantly (P<0.001) higher after induction of diabetes through STZ as matched to normal treated group. At 3 hrs, there was no significant (P>0.05) attenuation in blood glucose levels in animals group, which was treated with isolated compound (15.65 ± 0.34 mmol/L) as matched diabetic control group (16.15 ± 0.14 mmol/L), respectively. The attenuation in blood glucose levels starts after 6 hrs of receiving isolated compound as matched diabetic control group. The group IV diabetic rats that was treated with isolated compound at dose 50 mg/kg (b.wt) produced reduction in blood glucose level (15.10 ± 0.09; p< 0.05) after 6 hours of the treatment that became significant (P<0.01, P<0.001) (13.70 ± 0.09, 11.15 ± 0.25 mmol/L at 12 and 24 hours as matched to untreated diabetic control group (16.10 ± 0.29 mmol/L, 15.85 ± 0.35 mmol/L. Animals of group III that was treated with standard drug glibinclamide at dose of 20 mg/kg (b.wt) showed slightly (P<0.05) higher blood glucose levels at 3 hrs of treatment in comparing with that from of animals group (IV), which was treated isolated compound 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethyl-silylsalicylate at dose 50 mg/kg (b.wt). The standard animals treated group produced significant (P<0.01) reduction in blood glucose levels after 6 hrs of receiving the standard glibinclamide as matched to untreated diabetic and isolated compound (2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethylsilylsalicylate) group. The percentage in reduction of blood glucose levels in isolated compound animal group was (9.28%), (14.30%) and (28.20%) at 6, 12 and 24 hrs as matched to untreated diabetic control group.
| Treatment | Effect ofisolated compound and standard on blood glucose level in STZ-induced diabetic rats (mmol/L) | ||||
|---|---|---|---|---|---|
| 0 hr | 3hrs | 6 hrs | 12 hrs | 24 hrs | |
| Normal group | 5.2±0.05 | 4.55±0.14 | 4.80±0.20 | 5.00±0.40 | 4.55±0.64 |
| Diabetic group | 15.90±0.30 | 16.15±0.14 | 16.70±0.19 | 16.10±0.29 | 15.85±0.35 |
| Standard group | 16.45±0.45 | 16.30±0.09 | 15.10±0.09** (9.58%) |
12.35±0.15*** (9.28%) |
9.65±0.34*** (39.11%) |
| Isolated compound | 16.00±0.50 | 15.65±0.34 | 15.15±0.45* (9.28%) |
13.70±0.09** (14.30%) |
11.15±0.25*** (28.20%) |
Data are expressed as mean ± SEM, N=4, *P<0.05, Significant as matched to diabetic group, statistical test employed was two-way ANOVA followed by Bonferroni test
Table 2: Effect of isolated compound on blood glucose levels in STZ-induced diabetic rats
Discussion
The present research work was carried out on petroleum ether and ethyl acetate fraction of Pericampylus glaucus for isolation and characterization of compound and to evaluate the anti-diabetic activity of isolated compound. The present research work became the first documentary on active compound that was isolated from P. glaucus for hypoglycemic potential. Till to date, this compound was not reported for any pharmacological activities and not in literature were available concerning to their pharmacological and biological activities.
The use of allopathic anti-hyperglycemic medicines are not recommended always because of harmful toxic effects followed by unavailability and high cost.[28] Hence, an alternative source is required for the ailment of hyperglycemia and hyperlipidemia that are much safer and no harmful effect. The use of natural product for the remedy of diabetes and other health problem has long history that have been used from ancient time due lesser side effects and maximum benefits.[29] The compounds isolated from natural products continue to serve as viable source of naturally derived drugs for the world population are in extensive clinical use. Various compounds such as dehydrotrametenolic acid, isostrictinin, strictinin, roseoside, beta-pyrazol-1-ylalanine, epigallocatechin gallate, pedunculagin, cinchonain Ib, leucopelargonidin-3-O-alpha-L rhamnoside, leucocyandin 3-O-beta-d-galactosyl cellobioside, glycyrrhetinic acid and and christinin-A were isolated from different types of medicinal plants and have been shown significant antidiabetic potential along with insulinomimetic activity.[30] Terpeniods isolated from medicinal plants and have been reported for having antihyperglycemic activity due to stimulation of beta cell from pancreatic beta cell that will result secretion or stimulation of insulin.[31] A steroidal compound such as stigmasterol, which is isolated from the medicinal plant and has been reported for having significant hypoglycemic property in diabetic induced animal model.[32] A volatile compound such as “limonene” isolated from peel extract and may be a potential alternative for the treatment of hyperglycemia due to increasing glucokinase activity along with liver glycogen synthesis and decreasing plasma glucose and glycosylated hemoglobin levels.[33] On the basis of TLC, GC-MS followed by 1H NMR confirmed the compound and IUPAC name was 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethylsilylsalicylate. The single dose of isolated compound 2-[(trimethylsilyl)- oxy]-, methyl ester (cas) methyl o-trimethylsilyl-salicylate produced a significant attenuation in blood sugar level in diabetic animals. The animal group that was treated with isolated compound at a dose of 50.0 mg/kg (b.wt), reduced to the blood sugar level to 15.15 ± 0.45 mmol/L, 13.70 ± 0.09 mmol/L and 11.15 ± 0.25 mmol/L in 6, 12 and 24 hrs, respectively as matched to untreated diabetic control group. This falls in blood glucose level produced by isolated compound might be due to stimulation of the secretion of insulin from pancreatic beta-cells that promotes uptake of glucose metabolism and restore the remaining beta cells might be suggested the possible mechanism same as standard glibinclamide.[34] The standard drug, glibenclamide has been used for many years for treatment of type II diabetes mellitus, due to stimulation of beta cell of pancreas and thus caused the secretion of insulin from beta Islet of langerhans. It might be recommended that the mechanism of action of isolated compound 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethylsilyl-salicylate is analogue to standard oral hypoglycemic glibenclamide, this is could be the first report that reveals the antihyperglycemic properties for 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethylsilyl-salicylate.[35] The another possible mechanism in reduction of blood glucose level produced by isolated compound of P. glaucus may be due to potentiating effect on insulin by increasing either the insulin release from the bound form or secretion of insulin from pancreatic beta cells.[36] The glucose lowering effect produced by isolated compound might be also due to the release of high amount of insulin as the actions of oral hypoglycemic allopathic drug such as sulfonylurea’s.[37]
Conclusions
The present study confirmed the anti-hyperglycemic activity of compound 2-[(trimethylsilyl)-oxy]-, methyl ester (cas) methyl o-trimethylsilyl-salicylate isolated in STZ-induced diabetic rats. Our findings directly indicate that the isolated compound significantly attenuates the blood glucose level as compared with untreated diabetic group. This study justifies the use of plant in the remedy of diabetes mellitus and its connected problems. However, further pharmacological and biochemical investigations are needed to clearly elucidate the mechanism of action that will be helpful in projecting this plant as a therapeutic target in diabetes research.
Acknowledgements
The authors would like to acknowledge to the Faculty of Pharmacy, Lincoln University College, Malaysia, for facilitating instruments and laboratory supports to conduct the present study.
References
- Kifayatullah M, Sengupta P. Effect of Pericampylus glaucus on plasma glucose concentration and lipid profile in streptozotocin-induced diabetic rats. Bangladesh Journal of Pharmacology 2016;11:200-5.
- Kamboj A, Kumar S, Kumar V. Evaluation of antidiabetic activity of hydroalcoholic extract of cestrum nocturnum leaves in streptozotocin-induced diabetic rats. Advances in Pharmacological Sciences 2013;2013:354-62.
- Letchuman G, Wan Nazaimoon WM, Wan Mohamad WB, Chandran LR, Tee GH, Jamaiyah H, et al. Prevalence of diabetes in the Malaysian national health morbidity survey III 2006. Med J Malaysia 2010;65:180-6.
- Morbidity P. A Report of the Third National Health and Morbidity Survey 2015. Institute of Public Health, Ministry of Health Malaysia. 2015;1-48.
- Ho B, Jasvindar K, Gurpreet K, Ambigga D, Suthahar A, Cheong SM, et al. Prevalence, awareness, treatment and control of diabetes mellitus among the elderly: The 2011 National Health and Morbidity Survey, Malaysia. Malaysian Family Physician: The Official Journal of the Academy of Family Physicians of Malaysia 2014;9:1-315.
- Sharoni SKA, Wu SFV. Self?efficacy and self?care behavior of Malaysian patients with type 2 diabetes: a cross sectional survey. Nursing & Health Sciences 2012;14:38-45.
- Diabetes in Malaysia. International Diabetes Federation. 2015. Available on http://www.idf.org/membership/wp/malaysia [Lastaccessd on 25 october 2016].
- Sunil C, Latha PG, Suja SR, Shine VJ, Shyamal S, Anuja GI,et al. Effect of ethanolic extract of Pisonia alba Span. leaves on blood glucose levels and histological changes in tissues of alloxan-induced diabetic rats. International Journal of Applied Research in Natural Products 2009;2:4-11.
- Patel D, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific Journal of Tropical Biomedicine 2012;2:320-30.
- Kifayatullah M, Waheed I. Evaluation of hydroethanolic extract of Opuntia Monacantha Haw cladodes for antipyretic activity. World Journal of Pharmacy and Pharmaceutical Sciences 2014;3:1021-30.
- Kifayatullah M, Sengupta P, Mustafa MS, Das SK, Sisugoswomi M. Evaluation of Ethanolic Extract of Pericampylus glaucus (Lamk.) Merr for Total Phenolic, Total Flavonoids Contents and In-vitro Anti-Oxidant Activity. International Journal of Pharmacognosy and Phytochemical Research 2015;7:677-83.
- Ong H, Chua S, Milow P. Ethno-medicinal plants used by the Temuan villagers in Kampung Jeram Kedah, Negeri Sembilan, Malaysia. Ethno Med. 2011;5:95-100.
- Sigdel SR, Rokaya MB, Timsina B. Plant inventory and ethnobotanical study of Khimti Hydropower Project. Central NepalSci World 2013;11:105-112.
- Yan MH, Pi C, Jiang ZY, Yun-Bao M, Xue-Mei Z, Feng-Xue Z, et al. Periglaucines A-D, Anti-HBV andHIV-1 Alkaloids from Pericampylus glaucus. Journal of Natural Products 2008;71:760-3.
- Zhao WQ, Cui CB. Triterpenoidal constituents of Pericampylus glaucus and their antitumor activity in vitro. Chin J Med Chem 2009;19:195-9.
- Liang P, Zhou Q, Zhou F.Chemical constituents of Pericampylus glaucus (Lam.) Merr. Zhongguo Zhong Yao Za Zhi 1998;23:39-40,63.
- Natarajan V, Dhas ASAG. Effect of active fraction isolated from the leaf extract of Dregea volubilis [Linn.] Benth. on plasma glucose concentration and lipid profile in streptozotocin-induced diabetic rats. SpringerPlus 2013;4:394-400.
- Jamwal A. Systematic review on xanthones and other isolates from genus Swertia. Int J Pharm Chem Sci 2012;1:1464-82.
- Khaw LT, Leerach N, Yap NJ. A preliminary screening of potentially antimalarial plants against Plasmodium falciparum in vitro. Tropical Biomedicine 2015;32:676-83.
- Shipton FN, Khoo TK, Hossan M. Activity of Pericampylus glaucus and periglaucine: A in vitro against nasopharangeal carcinoma and anti-inflammatory activity. Journal of Ethnopharmacology 2017;198:91-7.
- KifayatullahM, Sarker MMR, Mustapha MS. Phytochemical investigation of ethanolic extract of Pericampylus glaucus leaves from Malaysia by GC-MS analytical technique. International Journal of PharmTech Research 2017;10:234-40.
- Dicke M, Van TA, Beek MA. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions Involvement of host plant in its production. Journal of Chemical Ecology 1990;16:381-96.
- Ezhilan BP, Neelamegam R. GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Res 2012;1:11-14.
- Ilani P, Ajodo N, Adewusi F. GC-MS and NMR analysis of the bioactive compounds from the crude extracts of Waltheria indica and the histopathological changes induced in albino rats challenged with Naja nigricollis venom. Journal of Coastal Life Medicine 2016;4:395-402.
- Kifayatullah M, Mustapha MS, Sengupta P. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. Journal of Acute Disease 2015;4:309-15.
- Kumar A, Ilavarasan R, Jayachandran T. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research. 2013;2:246-49.
- Candasamy M, Eswara T, Murthy GK. Alteration of glucose lowering effect of glibenclamide on single and multiple treatments with fenofibrate in experimental rats and rabbit models. Journal of Basic and Clinical Pharmacy 2014;5:62-68.
- Chawla R, Thakur P, Chowdhry A. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: a dreadful lifestyle disorder of 21st century. Journal of Diabetes &Metabolic Disorders. 2013;12:12-35.
- Modak M, Dixit P, Londhe J. Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition 2007;40:163-73.
- Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2012;2:320-30.
- Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res 2010;Article ID 483958.
- Panda S,Jafri M, Kar A, Meheta BA. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009;80:123-6.
- Murali R, Saravanan R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats.Biomed Prev Nutri 2012;2:269-75.
- Eliza J, Daisy P, Ignacimuthu S. Normo-glycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.) Sm. in streptozotocin-induced diabetic rats. Chemico-biological Interactions 2009;179:329-34.
- Kumar A, Ilavarasan R, Jayachandran T, Deecarama M. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research 2008;2:246-9.
- Fu Z, Gilbert ER, LiuD. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Current diabetes reviews 2013;9:25-53.
- Hosseyni ES, Kashani HH, Asadi MH. Mode of action of medicinal plants on diabetic disorders. Life Science Journal 2012;9:2776-83.