An Analysis of Pharmacological Management of Epilepsy and its Effect on Quality of Life
- *Corresponding Author:
- Dr. Prakruti Patel
Assistant Professor, Department of Pharmacology, BJ Medical College, Ahmedabad, India.
E-mail: prakrutiparth@yahoo.co.in
Citation: Patel P. An Analysis of Pharmacological Management of Epilepsy and its Effect on Quality of Life. J Basic Clin Pharma 2017;8:S044-S048.
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Objectives: The present study was undertaken to measure efficacy and tolerability of antiepileptic drugs in patients of epilepsy with a particular reference to the impact on quality of life (QOL). Methods: Adult patients of either gender, diagnosed with epilepsy who reported to the neuromedicine were enrolled after taking permission from Institutional Ethics Committee and were followed up for 6 months. The efficacy of the drug therapy was calculated by counting the number of seizure per month using seizure diary. QOL was measured using Quality of Life in Epilepsy-31-P questionnaire (QOLIE-31-p). Details of suspected adverse drug reactions, if any, were recorded. Statistical evaluation was done with unpaired t test, ANOVA and Pearson Parametric Correlation test. Results: Out of 120 patients, who completed the study 84 were already on drug therapy (OLD group), while 36 were started on drug therapy (NEW group). Sodium valproate was the most common drug prescribed as monotherapy as well as polytherapy. Drug therapy was significantly effective (p<0.001) in NEW group as mean number of seizures decreased from 1.69 ± 0.02/month to 0.25 ± 0.10 at end of six month. In QOL score increased from 50.16 to 70.21 in NEW group and 72.61 to 74.61 in OLD group. Pearson correlation (r) between QOL and efficacy for NEW group showed that QOL improved with decrease in mean number of seizure(r=-0.36). Conclusion Antiepileptic drugs are efficacious in treating and improving the quality of life of patients suffering from epilepsy.
Keywords
Antiepileptic drugs, QOLIE-31-p, quality of life, efficacy, cerebellar ataxia
Introduction
Epilepsy is a disease that effects people of all ages, though more frequently affecting young people in the first two decades of life and people over the age of 60.[1] Nearly 80% of epilepsy cases worldwide are found in developing regions.[2] Patients with epilepsy have poor healthrelated quality of life (HRQOL) and have 2 to 3 times more chance to have 14 or more physically unhealthy days in a year.[3] It also effect the social life of the patient for instance, driving vehicles, sports, etc.
Antiepileptic drugs are divided into conventional (phenytoin, phenobarbital, carbamazepine, sodium valproate, primidone, ethosuximide, clonazepam) and recently developed (gabapentin, lacosamide, lamotrigine, levetiracetam, rufinamide, riagabine, topiramate, zonisamide). Recent studies in both developed and developing countries have shown that up to 70% of newly diagnosed children and 60% of adults with epilepsy have been successfully treated (i.e., their seizures completely controlled) with anti-epileptic drugs (AEDs).
Efficacy of most antiepileptic drug is well established but their use may be associated with adverse effect which may also affect the quality of life. This may also influence the compliance of the patient. Quality of life is becoming an increasingly important object in assessing the overall success of the treatment. QOL is an important measure in epilepsy, which is often a chronic and debilitating condition and unique among the chronic illnesses due to the multidimensional impact on psychosocial functioning.[4] This study therefore aims to measure efficacy and tolerability of the commonly used antiepileptic drugs with a particular reference to the impact of these drugs on quality of life of the patient suffering from epilepsy.
Materials and Methods
This prospective longitudinal study with a follow up period of six months was carried out for a period of 22 months at the neuromedicine out-patient-department (OPD) of a tertiary care teaching hospital after approval from Institutional Ethics Committee (IEC). Permission to conduct the study was also obtained from medical superintendent and head of the department of neuromedicine. A written inform consent was also obtained from the patient after explaining them details of study.
Selection criteria
Patients of either gender aged between ≥ 18 years and ≤ 65, diagnosed with epilepsy who reported to the neuromedicine OPD of tertiary care teaching hospital and willing to participate were included in study. Patients with drug resistant epilepsy (a person has failed to become (and stay) seizure free with adequate trials of two antiepileptic drugs), major learning disability, previous diagnosis of psychiatric illness/nonepileptic seizure, pregnant women, lactating females, and patients with chronic diseases were excluded from study.
Study procedure
Patients who met with the selection criteria were enrolled and their general information, presenting complaints, past and family history were recorded. General examination, systemic examination and routine investigations were recorded at baseline as well as at 1st, 3rd and at 6th month of follow up. Investigation related to diagnosis of epilepsy if done, were recorded, which included Electroencephalography (EEG), Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI). At every follow up, presenting complaints or change in complaint were recorded. The efficacy of the drug therapy was calculated by counting the number of seizure per month. The patients were asked to maintain a seizure diary where the time and date of a seizure attack was recorded.
Quality of life was measured using Quality of Life in Epilepsy-31-P questionnaire (QOLIE-31-p) a validated questionnaire available in various languages after obtaining necessary permission from the author.[5] There are 38 questions about health and daily activities which are divided into seven subscales 1) Seizure worry 2) Overall QOL 3)Emotional wellbeing 4) Energy/fatigue 5) Cognitive functioning 6) Medication effects and 7) Social functioning. English, Hindi and Gujarati versions of the QOLIE-31-P were used for the study and the patient himself/herself completed the questionnaire. QOLIE-31-P questionnaire was administered at baseline, first follow up i.e., end of one month and at the end of last follow up i.e., at the end of six months. Quality of life scores were calculated using a score calculator as provided by the original source[5] where higher are score always reflect better quality of life. Details of suspected adverse drug reactions, if any, were recorded and analyzed by calculating their causality, severity and preventability.
Statistical Analysis
Statistical evaluation was done using GraphPad InStat Demo software, Unpaired t test, ANOVA and Pearson Parametric Correlation Test. Analysis of the suspected ADRs was done by WHO-UMC, Naranjo’s algorithm,[6] Hartwig and Siegel severity scale[7] and Modified Schumock and Thornton criteria for preventability.[8]
Results
This study was carried out for 22 months at neuromedicine OPD of a tertiary care teaching hospital in patients of epilepsy to study the efficacy and QOL. One thirty two patients were enrolled in our study (74 males and 58 females), out of which 12 were lost to follow up. Out of 132, patients 27 were from Ahmedabad, 64 from outside Ahmedabad but from Gujarat state and 41 from peripheral states mainly from Rajasthan and Madhya Pradesh. The age range of the patients was 18 to 65 years with a mean age 30.05 ± 9.60 years. A maximum of 54 patients (40.9%) were aged between 18 to 29 years. The mean weight of the enrolled patients was 50.03 ± 6.56 kg. None of the patients was illiterate as education up till 5th standard was a part of inclusion criteria. Most of the patients (64%) had education below or till 10th standard.
Most of the patients were housewives (33%), followed by patients who were unemployed (27%). Patients in this study largely belonged to medium socio-economic group (44%) with a yearly income between Rs. 40,000-80,000 followed by low income group (36%) with yearly income of less than 40,000 rupees. Fifty five patients reported that they had disturbed sleep, fourteen patients had irregular appetite while eight patients were addicted to smoking and seven to alcohol. Out of 120 patients, 84 were already on drug therapy at time of enrolment while 36 were started drug therapy for the first time and hence, were grouped as OLD group and NEW group respectively depending on treatment started. In OLD group while 61 patients (73%) came to OPD for follow up, 23 patients had complaint of seizure followed by aura (06 patients), fall (02 patients), and tongue bite (02 patients). In NEW group, all patients had chief complaint of seizure of which 15 patients also had aura, 4 had history of fall, and 6 patients history of tongue bite. Twenty seven patients had positive finding in the EEG, 12 on CT scan, 09 on MRI. All the symptoms were assessed at baseline and follow ups. It was observed that in NEW group patient’s post-treatment there was reduction in all symptoms viz. seizure, aura, fall, tongue bite at the end of 1st, 2nd and 3rd follow up as compared to baseline. At the time of enrollment, of study 128 out of 132 patients were labeled as GTCS. Four patients initially labeled partial seizure out of which two patients, later on were relabeled as Generalized Tonic Clonic Seizure during the course of study.
In OLD group, out of 84 patients, 32 were on monotherapy and 52 were on polytherapy whereas in NEW group, out of 36 patients, 26 were started on monotherapy and 10 patients on polytherapy. Sodium valproate was the most common drug prescribed as monotherapy as well as in polytherapy [Table 1].
| Drug therapy | OLD group(n=84) | NEW group(n=36) |
|---|---|---|
| Monotherapy Sodium valproate Phenytoin Carbamazepine |
32 15 11 06 |
26 14 07 05 |
| Polytherapy Valproate+phenytoin Phenytoin+Phenobarbital Carbamazepine+Sodium valproate Carbamazepine+clonazepam Carbamazepine+levitiracetam |
52 25 18 10 06 03 |
10 05 03 01 01 - |
Table 1: Drug therapy in patients of epilepsy
Efficacy of Antiepileptic’s was assessed by calculating the mean number of seizures per month after the start of therapy. Drug therapy was found to be significantly effective in NEW group as mean number of seizures per month decreased from 1.69 ± 0.02 at end of one month to 0.25 ± 0.10 at end of six month. As evident from Table 3 there was a significant decrease in the frequency of mean number of seizures at each month i.e., 2, 3, 4, 5, 6 months as compared to end of 1st month (p< 0.001). It was also observed that the mean number of seizure decreased significantly at 3, 4, 5, 6 months as compared to 2nd month (p< 0.05). In OLD group mean seizure at 1st month was 0.37 ± 0.07, 0.24 ± 0.05 at 2nd month and 0.22 ± 0.06, 0.24 ± 0.05, 0.24 ± 0.05, 0.22 ± 0.04 at 3rd, 4th, 5th and 6th months respectively. As evident from the above data, patient had low score from the first follow up itself. There was decrease in mean number of seizures per month at 2nd, 3rd, 4th, 5th and 6th months but this difference was not significant (p>0.05) [Table 2].
| Visits | OLD(Mean seizure ± SEM) | NEW(Mean seizure ± SEM) |
|---|---|---|
| (n=84) | (n=36) | |
| 1st Month | 0.37 ± 0.07 | 1.69 ± 0.20 |
| 2nd Month | 0.24 ± 0.05 | 1.08 ±0.20* |
| 3rd Month | 0.22 ± 0.06 | 0.66 ± 0.13*, ** |
| 4rd Month | 0.24 ± 0.05 | 0.44 ±0.10*, ** |
| 5rd Month | 0.24 ±0.05 | 0.27 ± 0.07*, ** |
| 6th Month | 0.22±0.04 | 0.25 ± 0.10*, ** |
Table 2: Efficacy of antiepileptic drugs in OLD and NEW group patient at different follow ups
For assessment of the quality of life, QOLIE-31P was used. In patients treated with antiepileptic drugs the baseline total score of quality of life was 65.65 ± 1.64. The energy score was 56.95 ± 1.37, mood was 64.73 ± 1.30, daily activities was 65.90 ± 1.48, cognition was 67.83 ± 1.88, medication effect was 77.51 ± 1.98, seizure worry was 63.34 ± 2.23 and overall quality of life score was 63.35 ± 1.57 (Mean ± S.E.M). As evident from the Table 3, compared to baseline, at 1st follow up (1st month) and at the end of last follow up (6th month), there was increase in all subscale of QOL and this difference was highly significant (p< 0.001). At baseline, in NEW group, mean score of overall quality of life was 50.16 ± 1.16, while in OLD group, it was 72.61 ± 1.43. As the months progressed there was an increase in QOL score of NEW group whereas QOL score of OLD group was high from baseline itself and remained similar over the follow up period. By the end of 6 months QOL score of NEW group was almost similar to OLD group patients [Table 4]. QOL of patients was also assessed according to age distribution. For this, patients were divided into three groups: 18 to 29 years; 30 to 39 years and 40 years or more. A slight decrease in QOL was observed with increase in age but difference was not significant (p>0.05). Males had higher QOL score compared to females but difference was not significant (p>0.05).
| Parameters | Baseline Score(mean ±S.E.M) | 1stfollow up(mean±S.E.M) | 2nd follow up(mean±S.E.M) |
|---|---|---|---|
| Energy | 56.95 ± 1.37 | 59.16 ± 1.32 | 63.87±1.03*,** |
| Mood | 64.73 ± 1.30 | 66.48± 1.26 | 71.8±1.04*,** |
| Daily Activities | 65.90 ± 1.48 | 67.86± 1.47 | 72.22±1.29*,** |
| Cognition | 67.83 ± 1.88 | 70.77 ± 1.82 | 76.77±1.61*,** |
| Medication Effect | 77.51 ± 1.98 | 70.50±1.90*** | 77.26±1.67**** |
| Seizure Worry | 63.34 ± 2.23 | 66.19± 2.15 | 73.56±1.96*,** |
| Overall Quality of Life | 63.35 ± 1.57 | 66.00± 1.47 | 71.22±1.13*,** |
| Total | 65.65 ± 1.64 | 66.70±1.34 | 72.38±1.42 |
Table 3: QOL in all the patients of epilepsy (n=120)
| Category | Visit | NEW (n=36) | OLD (n=84) |
|---|---|---|---|
| Energy | Baseline | 40.13 ± 0.98 | 62.15 ± 0.94 |
| 1st Follow up | 47.5 ± 2.31* | 62.84±1.69 | |
| 2nd Follow up | 63.19 ± 1.83** | 64.04 ± 1.43 | |
| Mood | Baseline | 48.88 ± 0.91 | 71.1±0.96 |
| 1st Follow up | 54.72 ± 2.01* | 71.2±1.03 | |
| 2nd Follow up | 71.44±2.00** | 72.8±1.10 | |
| Daily Activities | Baseline | 50.52 ± 1.37 | 70.3 ± 1.64 |
| 1st Follow up | 57.05 ± 2.58* | 71.6 ± 1.98 | |
| 2nd Follow up | 71.58±2.41** | 72.8 ± 2.00 | |
| Cognition | Baseline | 50.52 ± 1.21 | 75.3± 2.31 |
| 1st Follow up | 57.48 ± 1.29* | 74.9±2.56 | |
| 2nd Follow up | 75.8±2.10** | 77.23±39 | |
| Medication Effect | Baseline | - | 77.51 ± 1.98 |
| 1st Follow up | 58.53 ± 2.36 | 76.0 ± 2.36 | |
| 2nd Follow up | 76.1±2.21** | 77.5 ± 2.57 | |
| Seizure Worry | Baseline | 50.56 ± 2.26 | 72.4 ± 2.54 |
| 1st Follow up | 58.09 ± 2.29* | 72.5 ± 2.61 | |
| 2nd Follow up | 73.10±2.63** | 74.6 ± 2.70 | |
| Overall Quality of Life | Baseline | 50.16 ± 1.16 | 72.61 ± 1.43 |
| 1st Follow up | 56.76 ± 2.21* | 72.68± 1.52 | |
| 2nd Follow up | 70.21 ± 1.43** | 74.6± 1.37 |
Table 4: QOL score at baseline and follow up of NEW and OLD group
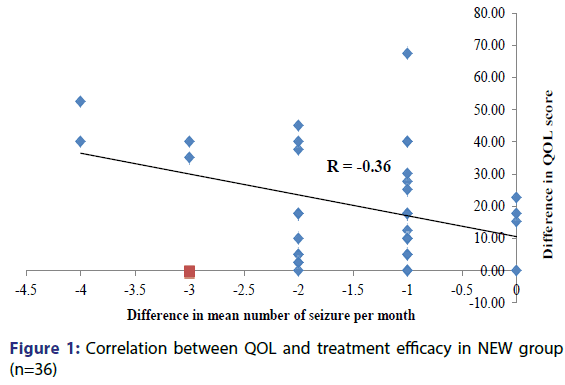
Correlation between QOL and efficacy of drugs was done for NEW group. Correlation was made using Pearson Parametric Correlation Test. Since the mean number of seizures per month decreased over the period of 6 months, the mean difference was negative and the correlation coefficient (r) calculated was -0.36. Thus, this correlation was significant (p< 0.05), which means that the decrease in the mean number of seizures per month strongly correlates with improvement in quality of life [Figure 1].
A total of 16 adverse drug reactions were reported during the study period. Out of these, rashes were caused by sodium valproate in 4 patients and by phenytoin in 3 patients. Three ADRs of cerebellar ataxia were found due to phenytoin, which was confirmed by clinical examination in 1 patient and by doing therapeutic drug monitoring of phenytoin level in 2 patients. So phenytoin was tapered and patients were shifted to sodium valproate. One ADR of dizziness due to carbamazepine was found. Phenytoin and phenobarbital given as polytherapy were found to cause rash in 2 patients and sedation in 2 patients. Sodium valproate and phenytoin given as polytherapy caused rash in 1 patient. As per WHO criteria, causality assessment was labeled as probable in 08 patients and possible in 08 patients whereas according to Naranjo’s scale 06 patients are labeled as probable and remaining 10 as possible. All the ADRs were found to be probably preventable type according to modified Schumock and Thornton’s criteria. As per severity assessment (modified Hartweig and Seigel scale), 13 patients had mild ADR and 03 patients of cerebellar ataxia had ADR of moderate severity.
Discussion
Epilepsy is a neurological condition that effect people of all geographic, social or racial class. Antiepileptic drugs are the treatment of choice for epilepsy. Antiepileptic drugs are able to alleviate the symptoms but may produce adverse reactions which may also affect the quality of life in epilepsy patients. Therefore, measurement of quality of life is considered vital for the care of epileptic patients.
The mean age of the patients in our study was 30.05 ± 9.60 years. Our study shows a higher prevalence of epilepsy in males as compared to females (M:F=74:58). None of the patient in our study was illiterate as minimum 5th standard of education was inclusion criteria as it was the minimum education required to comprehend questions of QOL however 64% of patients had education either till primary or secondary level only as ours is a government hospital where patients drained from peripheral parts, where have poor education. A total of 27% patients were unemployed in our study. This high rate of unemployment is because patients with epilepsy have more chances of leaving school in early life and thus fail to undergo subsequent training or apprenticeships. Contrary to this in other studies number of unemployed patients were even higher being 65.6% in Brazilian study,[10] 68.9% in Malaysian study,[9] and 44.5% in a South Indian study.[11] Patients in our study largely belonged to medium socioeconomic group followed by low income group. In India most of the health care system is private which is used by most of the affordable patients and our study has been conducted in a government hospital where treatment is given free of cost or at a very low price, thus catering a large number of patients belonging to low socio-economic status.[12]
Forty six percent patients had irregular sleep of which 25% were from NEW group. Evidence suggests that having epilepsy and the occurrence of seizures, as well as some AEDs, are associated with significant sleep disruption. (The Neurological Institute, NEW York). Wells[13] has observed that seizures are common during sleep in certain epileptic syndromes and occur only during NREM sleep while studies also suggest that sleep deprivation can also precipitates seizures. So in patients with disturbed sleep and epilepsy, treatment of epilepsy as well as sleep disorder will improve the patient’s overall health. Such patients will require an additional psychological therapy to achieve overall improvement in QOL.
In OLD group out of 84 patients 32 were on monotherapy and 52 were on polytherapy whereas in NEW group out of 36 patients 26 were started on monotherapy and 10 patients on polytherapy. According to NICE[14] and ILAE treatment guidelines any newly diagnosed patient should be started on monotherapy and if it fails then only polytherapy be considered but in our study 10 NEW patients were started on polytherapy which is not justifiable. In NEW group sodium valproate was the most common antiepileptic drug prescribed as monotherapy followed by phenytoin and carbamazepine. While evaluating the individual drugs prescribed in OLD group also observed similar results in which sodium valproate was the most common antiepileptic drug prescribed as monotherapy. As per both NICE[14] and ILAE[15] treatment guidelines also sodium valproate is the preferred first line drug followed by lamotrigine for GTCS. In our study instead of lamotrigine, phenytoin was the 2nd most common drug since ours is a government setup, and lamotrigine being a newer drug is not available.
Two patients were advised serum phenytoin level (TDM) after suspicion of cerebellar ataxia and after positive laboratory finding both of them were shifted to sodium valproate where as one patient was shifted on sodium valproate on the basis of positive sign and symptoms of phenytoin toxicity. In our study TDM is not common as this facility is not available in our hospital setup and is not done to check the compliance of drug therapy. It is done from private laboratory when a serious ADR due to drug toxicity is suspected. However, availability of such facility can help to monitor the drug level for better treatment efficacy.
Efficacy of drug therapy was assessed by calculating the mean number of seizures per month after start of therapy. Drug therapy was found to be significantly effective in NEW group as mean number of seizures per month decreased. Our study had the limitation that follow was for only 6 months whereas most of the efficacy studies require a longer follow up but we could establish statistically significant reduction in mean number of seizures even over a period of 6 months in patients who were newly started on drugs. In our study mean number of seizure was 0.37 ± 0.07 at the end of one 1 month and 0.22 ± 0.04 at the end of 6th month in the OLD group. There was no significant reduction in mean seizure over follow up period as patients already had lower value from the baseline itself suggesting a good control of therapy on seizures. So we can establish that long term therapy is efficacious and maintains good control over seizure frequency.
While most of the studies conducted on QOL in epilepsy throughout the world are cross-sectional our study had the advantage of having follow up of 6 months from time of enrolment. On follow up we noted that in NEW patients there was a significant improvement in QOL score from 50.16 ± 1.16 at baseline to 70.21 ± 1.43 at end of follow up while in OLD patients had significantly high QOL score from baseline (72.61 ± 1.43) itself which was maintained throughout the follow up (74.6 ± 1.37) period. Even after extensive research we not able to find a follow up study for QOL in patients of epilepsy treated medically. So our study is a de-novo study with follow up period in which we observed a significantly improved QOL in NEW patient, helping us conclude that QOL in epilepsy significantly improves with the use of antiepileptic drugs. In our study we noted that males had higher QOL score compared to females as it was found that male enrolled in study had higher education than females, thus contributing to better QOL. We also noted that QOL score was found to decrease with increasing age most probably due to chronicity of disease and drug therapy thus effecting QOL.
In our study showed a significant (p < 0.05) correlation between quality of life and treatment efficacy which means that decrease in the mean number of seizures per month strongly correlates with improvement in quality of life. Thus, antiepileptic drugs which are effective in reducing the mean number of seizures improve QOL. Thus seizure frequency is an important assessment tool for evaluating efficacy and to find a correlation between efficacy and QOL.
A total number of 16 adverse drug reactions were reported during this study. Majority of the ADRs were mild and probably preventable. 3 ADRs of cerebellar ataxia due to phenytoin needed replacement by Na valproate. However, under-reporting due to poor educational status cannot be ruled out.
Our study had some limitations too. A large number of patients and a longer follow up would give us a better idea about disease and impact of the drug therapy on quality of life. The prescription of drug therapy was restricted by the drugs in hospital supply as study was done in a government institution. However, importance of the present study cannot be undermined. It is one of the few studies conducted in India on quality of life in patients of epilepsy and is the first study done with a follow up period in patients of epilepsy. Also, it is one of the few Indian studies that have attempted to correlate between quality of life and severity of epilepsy in terms of frequency of seizures. A very significant correlation between quality of life and seizure frequency has also been noted.
The study concludes that antiepileptic drugs are efficacious in treating and improving the quality of life of patients suffering from epilepsy. Hence, the choice of therapy depends on the cost of therapy as well as availability and tolerability of the drug in public health care setup. It is recommended that besides providing an optimum drug treatment that relieves the current symptoms of the disease, quality of life should also be considered as a prognostic tool and attempts should also be made to provide a psycho-social support to obtain a significant improvement in quality of life. We also recommend the use of a seizure diary as a part of regular therapy to get better follow up on the prognosis of disease, efficacy of on-going treatment and to correlate its effect on QOL.
Bullet points
1. A total of 132 patients were enrolled, out of which, 120 patients completed the study, and male to female ratio was 4:3. These patients were divided into two groups, one of fresh cases (NEW) and second of patients already taking antiepileptic drugs (OLD). Of the 120 patients 84 were OLD and 36 were NEW. The mean age of patients was 30.05 ± 9.60 years and mean weight of the patients was 50.03 ± 6.56 kg. About two third (65%) of the patients had education in range of 5th to 10th standard. Majority of the patients (44%) belonged to medium socio-economic group. It was observed that out of 120 patients, 55 had irregular sleep and 14 had irregular appetite.
2. Efficacy of drug therapy was assessed by calculating the mean number of seizures per month after start of therapy. Patient was asked to keep a seizure diary in which patient used to note down every seizure attack with date and time during the whole follow up of 6 months. In NEW group it was observed that there was a decrease in the frequency of mean number of seizures at each month as compared to 1st month (p< 0.001). Whereas in OLD group as the patient were already on drug therapy at the time of enrolment the seizure frequency was already lower than the NEW group at baseline.
3. For assessment of the quality of life, seven different parameters were calculated using QOLIE-31-P: 1) Seizure worry 2) Overall QOL 3) Emotional wellbeing 4) Energy/fatigue, 5) Cognitive functioning 6) Medication effects and 7) Social functioning. In 120 (OLD+NEW group) patients at the end of 2nd follow up there was increase in scores of all the parameters compared to baseline which was highly significant (p< 0.001). In NEW group overall score of QOL was significantly high at second follow up as compared to the baseline and first follow up (p< 0.001) while in OLD group baseline score were already high and no significant increase in scores were observed on follow up.
4. In NEW group a correlation between quality of life score and treatment efficacy i.e., mean number of seizure was made by Pearson Parametric Correlation Test. Since the mean number of seizures per month decreased over the period of 6 months, the mean difference was negative and the correlation coefficient (r) calculated was -0.36. Thus, this correlation was significant (p< 0.05), which means that decrease in the mean number of seizures per month correlates with improvement in quality of life.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure
Dr. Mradul gupta has received support from Dr. Prakruti P. Patel. The remaining authors have no conflicts of interest.
References
- Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol 2003;16:165-70.
- World Health Organization. Epilepsy: Fact sheet 2015. Available from http://www.who.int/mediacentre/factsheets/fs999/en/
- Cardarelli JW, Smith JB. The burden of epilepsy to patients and payers. Am J Manag Care 2010;16:331-6.
- Baker GA. Quality of life and epilepsy: the Liverpool experience. Clin Ther 1998;20:2-12.
- Cramer JA, Hammee VG. N132 Study Group. Maintenance of improvement in health-related quality of life during long-term treatment with levetiracetam. Epilepsy and Behaviour 2003;4:118-23.
- Naranjo CA, Busto U, Sellers EM, Sandor P. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
- Hartwig S, Seigel J.Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229-32.
- Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992;27:538.
- Vanitha Rani N.Frequent seizures and polytherapy can impair quality of life in persons with epilepsy. Int J Res Pharm Sci 2013;4:141-5.
- Tegegne TM, Muluneh NY, Wochamo TT. Assessment of Quality of Life and Associated Factors among People with Epilepsy Attending at Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia. Sci J Public Health 2014;5:378-83.
- Salgado PCB, Cendes F. The effects of Epileptic Seizures upon Quality of Life. J Epilepsy Clin Neurophysiol 2009;15:110-3.
- Elwes RD, Marshall J, Beattie A, Newman PK. Epilepsy and employment. A community based survey in an area of high unemployment. J Neurology, Neurosurgeryand Psychiatry 1991;54:200-3.
- Wells ME. Epilepsy: The night thief. Available from https://go.aastweb.org/Resources/A2Zzz/Literary%202010%20Wells.pdf
- National Institute for Health and Care Excellence. Epilepsies: diagnosis and management 2012. Available from https://www.nice.org.uk/guidance/cg137
- Pathogenesis of GTCS. The Epilepsies: Seizures, Syndromes and Management: Based on the ILAE Classifications and Practice Parameter Guidelines, 2005.
- Czuczwar SJ, Kaplanski J, Swiderska-Dziewit G. Pharmacodynamic interactions between antiepileptic drugs: preclinical data based on isobolography. Expert Opin Drug Metab Toxicol 2009;5:131-6.
- Mane YV, Potey A, Bhide SS. Drug utilization pattern of antiepileptic drugs and direct and indirect cost estimation in the treatment of epilepsy at tertiary care hospital. IntJInform Res Rev2015;2:759-64.
- Heller AJ, Chesterman P, Elwes RDC.Phenobarbitone, phenytoin, carbamazepine or sodium valproate for newly diagnosed adult epilepsy: a randomized comparative monotherapy trial. J Neurology, Neurosurgery and Psychiatry 1995;58:44-50.
- Djibuti M, Shakarishvili R. Influence of clinical, demographic, and socioeconomic variables on quality of life in patients with epilepsy: findings from Georgian study. JNeurology, Neurosurgery and Psychiatry 2003;74:570-3.
- Pal AA, Prusty SKA, Sahu PKA. Drug utilization pattern of antiepileptic drugs: A pharmacoepidemiologic and pharmacovigilance study in a tertiary teaching hospital in India. Asian J Pharma Clin Res 2011;4:96-9.
- S RB, Narayan SS, Sharma GRK, Rodrigues, RJ. Pattern of adverse drug reactions to anti-epileptic drugs: a cross-sectional one-year survey at a tertiary care hospital. Pharmacoepidem. Drug Safe 2008;17:807-12.